Pectic oligosaccharides from cranberry prevent quiescence and persistence in the uropathogenic escherichia coli cft073
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Urinary tract infections (UTIs) caused by _Escherichia coli_ create a large burden on healthcare and frequently lead to recurrent infections. Part of the success of _E. coli_ as an
uropathogenic bacterium can be attributed to its ability to form quiescent intracellular reservoirs in bladder cells and its persistence after antibiotic treatment. Cranberry juice and
related products have been used for the prevention of UTIs with varying degrees of success. In this study, a group of cranberry pectic oligosaccharides (cPOS) were found to both inhibit
quiescence and reduce the population of persister cells formed by the uropathogenic strain, CFT073. This is the first report detailing constituents of cranberry with the ability to modulate
these important physiological aspects of uropathogenic _E. coli_. Further studies investigating cranberry should be keen to include oligosaccharides as part of the ‘active’ cocktail of
chemical compounds. SIMILAR CONTENT BEING VIEWED BY OTHERS LYOPHILIZED CELL-FREE SUPERNATANTS OF _LIMOSILACTOBACILLUS FERMENTUM_ T0701 EXHIBITED ANTIBACTERIAL ACTIVITY AGAINST _HELICOBACTER
PYLORI_ Article Open access 13 June 2024 ANTIBACTERIAL EFFICACY OF BERRY JUICES AGAINST _BACILLUS CEREUS_ RELATIVE TO THEIR PHYTOCHEMICAL COMPOSITION AND ANTIOXIDANT PROPERTIES Article Open
access 16 November 2024 COMPARATIVE METABOLITE PROFILING OF FOUR POLYPHENOL RICH MORUS LEAVES EXTRACTS IN RELATION TO THEIR ANTIBIOFILM ACTIVITY AGAINST _ENTEROCOCCUS FAECALIS_ Article Open
access 23 November 2022 INTRODUCTION Bacterial urinary tract infections (UTIs) affect up to 150 million people each year and are among the most common infections worldwide1. It is estimated
that the total societal cost burden of UTIs is $3.5 billion each year in the US alone2. Over 50% of women will experience a UTI, with an estimated recurrence rate of about 25%3. While UTIs
are typically curable, recurrent infections can require continuous antibiotic prophylaxis for prevention. Frequent use of antibiotics, however, can lead to adverse events such as colitis,
the development of antibiotic resistant UTIs, or the development of complicated UTIs2,3,4. Uropathogenic _Escherichia coli_ (UPEC) is responsible for 65–90% of UTI cases2,4,5,6. For
recurrent UTIs, the original infecting UPEC strain is responsible 77% of the time7. While the mechanism of recurrence is not fully understood, it is thought that intracellular, biofilm-like
communities (IBCs) and quiescent intracellular reservoirs (QIRs) are contributing factors8,9,10. IBCs form when UPEC from urine invade and replicate inside of superficial bladder cells6,10.
As the infection progresses, subsequent UPEC invasion of urothelial transitional cells can lead to establishment of QIRs. Cells residing in QIRs can remain viable for months6,8,9. Upon
exfoliation of bladder epithelial cells, UPEC from QIRs would be able to re-colonize urine and cause a recurrent infection6. Since antibiotics generally target actively dividing bacteria,
quiescent UPEC cells are unlikely to be eradicated by antibiotics, thus contributing to treatment failures and recurrence8,10. Persistence is a physiologic state similar to quiescence for
UPEC11,12,13,14. Persister cells are a small subpopulation of bacteria that exist in a dormant, non-dividing state characterized by high antibiotic tolerance11,12,14. Persister cells can be
isolated and regrown following initial antibiotic treatment, and these populations retain sensitivity to the initial antibiotic and can consistently form another persister cell fraction15.
Similar to QIR cells, persister cells are strongly implicated in the pathogenesis of chronic infections, including recurrent UTIs16,17,18,19,20. While proven to be metabolically distinct,
the quiescent and persistent physiological states of UPEC are both likely to cause recurrent UTIs and treatment failures16. Therapies aimed at preventing or reversing quiescence and
persistence could significantly reduce the burden of UPEC recurrent UTIs. Cranberry (_Vaccinium macrocarpon_) products have been extensively researched for their ability to prevent UTIs,
especially those caused by UPEC21,22,23,24,25,26. Past studies have focused on cranberry proanthocyanidins (PACs) with A-type linkages24,27 and fructose28 since these have been shown to
interfere with UPEC adherence to bladder cells. Organic acids present in cranberry, including quinic, shikimic, malic and citric acids, were recently demonstrated to help reduce _E. coli_
populations in an experimental mouse model of UTI29. Other recent studies have suggested that cranberry complex carbohydrates might also contribute to the prevention of UTIs. For example,
xyloglucan oligosaccharides from cranberry have been shown to interfere with cell adhesion and biofilm formation by uropathogenic _E. coli_30,31,32. We hypothesized that certain cranberry
constituents might interfere with quiescent and persister cells since these are implicated in UTI recurrence. Herein, we report the isolation and chemical characterization of cranberry
pectic oligosaccharides (cPOS) that prevent quiescence in the prototypical UPEC strain CFT073 and significantly reduce the population of UPEC persister cells in the presence of ampicillin.
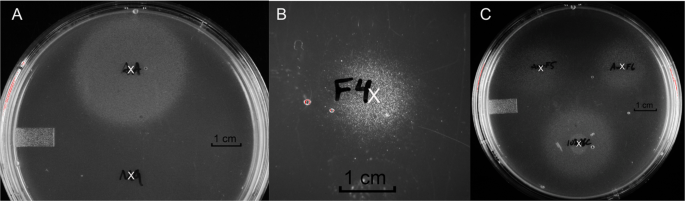
RESULTS THE UPEC STRAIN CFT073 DEMONSTRATES _IN VITRO_ QUIESCENCE ON GLUCOSE MINIMAL MEDIA AGAR PLATES AND RESUMES GROWTH IN RESPONSE TO CRANBERRY CONSTITUENTS We previously reported that
the UPEC strain CFT073 grows normally in liquid glucose minimal media but exhibits _in vitro_ quiescence on glucose minimal media agar plates when plated at low density (≤106 CFU)16. It was
found that cells exhibiting quiescence under these conditions could be stimulated to grow by transferring a colony of _E. coli_ MG1655 onto the plate or by applying an amino acid cocktail
comprising various combinations of lysine, methionine, and tyrosine. Following addition of the stimulus to the agar surface and 24 hour incubation at 37 °C, growing CFT073 cells appear as
visible colonies radiating out from the site of the stimulus (see Fig. 1 as an example). The diameter of the zone of growing CFT073 continues to expand with additional incubation time. This
experiment is a qualitative test for stimuli that reverse _in vitro_ quiescence of UPEC strains. Because cranberry has been traditionally used for preventing UTI, we hypothesized that
certain cranberry constituents might reverse UPEC quiescence. The above quiescence assay was used to test cranberry phytochemicals and guide the separation of the active components (Fig.
S1). A pectinase degraded cranberry hull extract was separated using C18 reversed phase chromatography to afford an oligosaccharide-enriched fraction, Cranf1b, that induced bacteria colony
formation when added (100 µg in 10 µL water) to the surface of an agar plate containing quiescent CFT073 (data not shown). Cranf1b was further separated using porous graphitized carbon (PGC)
cartridges. Only a sub-fraction that eluted with 10% ACN/H2O/0.1%TFA showed reversal of _E. coli_ CFT073 quiescence (Fig. 1). This sub-fraction (cPOS) was further separated using HPLC
chromatography and characterized using a combination of NMR, HR-MS and LC-MS/MS. CHEMICAL CHARACTERIZATION OF CRANBERRY CONSTITUENTS THAT REVERSE QUIESCENCE The 1H and HSQC NMR spectra of
the active cranberry fraction revealed proton and carbon resonances with chemical shifts characteristic for poly-uronic acids. Additional structural features included a methoxy group at δ
3.70/52.8 and a vinylic methine at δ 6.00/111.8 (Figs. S2 and S3). Monosaccharide composition analysis of cPOS determined that the oligosaccharides were comprised of galacturonic acid units
(Fig. S4). The active fraction was further analyzed by hydrophilic interaction liquid chromatography (HILIC) HPLC (Fig. 2). HILIC LC-MS data showed that the predominate ions were consistent
with poly-galacturonic acid methyl esters with various degrees of polymerization (DP) (Fig. 3). Each oligosaccharide was present as a pair of α- and β- anomers in equilibrium. These results
were in agreement with HR-ESI-MS and HR-ESI-MS/MS data obtained for collected fractions (Figs. S5 and S6). The most abundant ions indicated the presence of fragments with one or two free
carboxylic acids and one additional degree of unsaturation. UV absorption maxima at 235 nm was consistent with the presence of an α, β-unsaturated carboxylic acid, which is in agreement of
the vinylic methine observed in NMR, and accounting for the additional unsaturation. The major components were therefore determined to be unsaturated pectic oligosaccharides, referred to as
uGXmY (uG: unsaturated poly-Galacturonic acid; X: degree of polymerization; Y: number of methyl esters). Saturated poly-galacturonic acid methyl esters were unexpectedly found to be present
only trace amounts in cPOS (data not shown). We next isolated two unsaturated poly-galacturonic acid methyl esters, uG3m2 (HRMS (_m/z_): [M−H]−, C20H27O18, 555.1205, calcd. 555.1197) and
uG4m3 (1, HRMS (_m/z_): [M−H]−, C27H37O24, 745.1692, calcd.745.1674) from the cPOS mixture. Compounds eluting after 1 were pooled into a single fraction containing cPOS with higher DPs
(HDP-cPOS). Purified 1 was characterized using 1D and 2D NMR spectra (Figs. S7–S12) and compared to previously reported poly-galacturonic acids33,34,35,36. Superimposition of HSQC spectra
for both 1 and cPOS showed that both contained similar correlation signals (Fig. S3), confirming 1 as a representative constituent of cPOS. The 1H and 13C NMR chemical shifts of 1 are listed
in Table S1. Briefly, five spin systems were identified and were assigned to the four subunits (A-α/A-β, B, C, D) of 1 (Fig. 4). Key HMBC and NOE correlations are shown in Fig. 4. HMBC
correlations between the methoxy protons and the C-6 carbonyls were observed for subunits A, B and D, indicating that the free carboxylic acid was located on subunit C. The C-6 of subunit C
also has a higher NMR chemical shift (δ 175.0 ppm), consistent with a carboxylic acid at this position. The MS/MS fragmentation patterns indicated sequential neutral losses of 190 Da for 1
(Fig. 5), further supporting the structure. Three previously reported iridoid glucosides were isolated from the cPOS fraction as minor impurities and identified as 6,7-dihydromonotropein,
deacetylasperulsidic acid and monotropein37,38,39,40. These iridoid glucosides were inactive in the _in vitro_ quiescence inhibition assay (data not shown). We found that these could be
removed from the cPOS fraction by exploiting their differential solubility in ethanol. Cranf1b was triturated with 95% aqueous ethanol prior to PGC separation, producing a cPOS fraction
(cPOS-t) devoid of iridoid glycosides as determined by HPLC analysis (Fig. 2) and LC-MS ion extraction (data not shown). PURIFIED OLIGOSACCHARIDES UG3M2 AND UG4M3 INHIBIT _E. COLI_ CFT073
QUIESCENCE The isolated oligosaccharides uG4m3 and uG3m2 were tested alongside cPOS and HDP-cPOS for reversal of _E. coli_ CFT073 quiescence (Fig. 1). All four samples, applied as 100
μg/10μL aqueous solutions to the agar surface, stimulated the growth of quiescent bacteria, indicating an inhibition of quiescence. The quiescence assay was repeated using glucose-free
plates to test if bacteria growth was simply due to bacteria using the added oligosaccharides as a carbon source. The addition of uG3m2 (100 μg/10μL) onto glucose-free plates containing
quiescent CFT073 did not result in visible growth of bacteria colonies (Fig. 6). However, co-spotting 100 μg uG3m2 and 2 mg glucose onto agar containing quiescent CFT073 stimulated bacteria
growth. This result is consistent with the cranberry oligosaccharides stimulating the quiescent UPEC to grow on glucose. _E. COLI_ CFT073 GENERATES A LOW LEVEL OF PERSISTER CELLS IN LIQUID
GLUCOSE M9 MINIMAL MEDIUM CONTAINING CRANBERRY PECTIC OLIGOSACCHARIDES Normal microbial populations contain persister cells as small subpopulations (10−3 to 10−4%) of dormant cells that are
tolerant to antibiotics. Upon regrowth in the absence of the drug, persister cells produce cultures that regain full sensitivity to the antibiotic. Persister cells likely play a role in
chronic infections13. We previously found that _E. coli_ CFT073 generates high levels of persister cells in liquid glucose M9 minimal medium in the presence of ampicillin (100 µg/mL), and
the addition of a mixture of the 20 standard L-amino acids (100 µg/mL each) reduced persister cell populations by 100-fold during ampicillin treatment16. Since cPOS reversed _E. coli_ CFT073
quiescence on glucose plates, we were interested in determining whether cPOS addition to cultures of _E. coli_ CFT073 grown in glucose M9 minimal medium in the presence of ampicillin would
generate fewer persister cells. Overnight cultures of _E. coli_ CFT073 grown on 0.4% glucose M9 minimal medium were diluted 20-fold into fresh 0.2% glucose M9 minimal medium (_A_600 of 0.1,
~108 CFU/mL) containing or lacking ampicillin (100 µg/mL), and viable counts were measured after 4 h and 24 h at 37 °C (Fig. 7). As previously observed16, the viable cell counts in the _E.
coli_ CFT073 cultures decreased slightly during the first 4 h of incubation in both the presence and absence of ampicillin. By 24 h, _E. coli_ CFT073 had grown to just over 109 CFU/mL in the
absence of ampicillin (Fig. 7). In contrast, the presence of ampicillin reduced the viable counts an additional ~1000-fold over the next 20 h, to about 106 CFU/mL. As shown by the results
in Fig. 7, in the presence of 1 mg/mL cPOS and absence of ampicillin, _E. coli_ CFT073 viable counts increased rapidly, reaching stationary phase within 4 h. Importantly, in the presence of
both the cPOS and ampicillin, _E. coli_ CFT073 viable counts decreased rapidly within 4 h, and reached a level of about 3 × 102 CFU/mL after 24 h (Fig. 7). Therefore, treating CFT073 with
both cPOS and ampicillin resulted in >1000-fold fewer persister cells versus treatment with ampicillin alone. Similar reduction of persisters was obtained when conducting the assay with
cPOS-t (Fig. S13). DISCUSSION The oligosaccharides discussed in this manuscript suggest previously undiscovered benefits of cranberry constituents for mitigating UPEC infections. Other than
specific amino acid cocktails, cPOS represents the first molecules shown to reverse a quiescent phenotype and reduce a persister cell fraction in the UPEC strain CFT073. Inhibition of UPEC
quiescence is intriguing since this phenotype has been linked with recurrent UTIs6. Persistence and quiescence are both phenotypes involving dormant bacteria cells; however, it remains to be
determined if the physiologic change(s) induced in UPEC by cPOS is shared between the two cell types. Prevention of UPEC dormancy may allow host defense mechanisms and common treatment
approaches to be more effective. CFT073 is a prototypic UPEC strain belonging to the phylogenetic group B2 multilocus sequence type 73 strains (ST73). ST73 is a major UPEC lineage,
accounting for 11% and 16.6% of UPEC isolated from patients in recent studies41,42. It was previously shown that CFT073 enters a quiescent state when plated onto M9 minimal medium agar
plates containing glucose at concentrations ≤106 CFU16. A recent study found that 30 of 38 ST73 strains (78.9%) entered the quiescent state on M9 glucose plates, demonstrating that
quiescence is a common phenotype for this UPEC lineage. UPEC strains belonging to other sequence types (ST141, ST104, ST394, and ST998) have also demonstrated the same _in vitro_ quiescent
phenotype16. Additional studies are underway to determine if cPOS reverses quiescence in UPEC strains other than CFT073. We have shown that cranberry pectic oligosaccharides reduce a
persister cell fraction of the UPEC strain CFT073 formed in the presence of ampicillin. Ampicillin belongs to the beta-lactam class of antibiotics and inhibits enzymes critical for cell wall
biosynthesis. Further studies are needed to determine if cPOS similarly reduces persister cell fractions when used with other antibacterial agents, especially those that inhibit bacteria
via other mechanisms of action such as the fluoroquinolones. The dehydration on subunit D is likely due to the enzymatic treatment used to break down insoluble pectic polysaccharides during
cranberry juice manufacturing33. Some pectinases can selectively hydrolyze glycosidic bonds by eliminative cleavage and cause oxygen-aglycone bond breakage43,44,45. For example, a
mechanistic study on a pectin lyase A (PLA) derived from _Aspergillus niger_, of the same origin as the Klerzyme 150 in this study, showed specific cleavage of a glycosidic bond adjacent to
a methyl-esterified galacturonic acid residue, leaving a 4,5-unsaturated galacturonic acid methyl ester at the non-reducing end46. Interestingly, it was also pointed out that free carboxylic
acids generally occurred second from the non-reducing end, which is in agreement with our assignment of 146. Previous studies of cranberry oligosaccharides have focused on xyloglucans and
their antibiofilm and antiadhesion properties30,31,32. A recent report showed that xyloglucans can be found in pig urine following oral administration31. In the study presented here,
xyloglucans were separated from cPOS by PGC chromatography and were inactive when tested in the quiescence and persister cells assays. Hence, there may be distinct health benefits from the
structurally different oligosaccharide fractions found in cranberry. The results presented here encourage further investigation of cPOS as agents to combat recurrent _E. coli_ UTIs and
support current drug treatment options. Our findings complement those concerning other cranberry constituents (e.g. PACs, organic acids and xyloglucans) and their possible benefits in
preventing UTI infections. It is rational to speculate that the full benefit of cranberry may not be realized with one chemical class, but rather that multiple classes act in concert.
Reviews of the published clinical data show inconsistencies in chemical characterization and preparation of cranberry products tested for UTI prevention22. The optimal formulation and use of
cranberry products for UTI prevention will require further knowledge of the active constituents and mechanisms of action. Until then, cranberry juice or whole berry products might be best
used until more is known regarding how and why cranberry may aid in preventing UTIs. METHODS GENERAL EXPERIMENTAL PROCEDURES NMR experiments were conducted using an Agilent Inova NMR 500 MHz
spectrometer in D2O (99.99%, Sigma-Aldrich, St. Louis, MO, USA) at 25 °C. LC-MS analysis was performed on a Prominence UFLC system (Shimadzu, Kyoto, Japan) coupled to a QTRAP 4500 mass
spectrometer (AB Sciex, Framingham, MA, USA) with electrospray ionization source on negative ion mode with scans from _m/z_ 200 to _m/z_ 2000. High Resolution Electrospray Ionization mass
spectra and tandem mass spectra (HR-ESI-MS and HR-ESI-MS/MS) were acquired using a TripleTOF 4600 spectrometer (AB Sciex) operating in negative ion mode. Flash chromatography was completed
using an Agilent 971-FP flash purification system (Agilent Technologies, Santa Clara, CA, USA) with Biotage SNAP KP-C18-HS 120 g cartridges (Biotage, Charlotte, NC, USA). HPLC separations
were performed on a Shimadzu Prominence i-series HPLC (Shimadzu). SEPARATION OF CRANBERRY PECTIC OLIGOSACCHARIDES Cranberry hulls were degraded with pectinase (Klerzyme 150, DSM Food
Specialties, Heerlen, Netherlands) and fractionated as previously described with modifications30. Briefly, cranberry pectinase treated hull extract was fractionated using a C18 flash column
sequentially eluted with 500 mL 100% deionized (d.i.) water, 500 mL 15% methanol/H2O and 500 mL 100% methanol. Fractions from each step-gradient were individually pooled into three major
fractions, Cranf1W (38.1%, w/w, dry), Cranf1b (23.8%, w/w, dry) and Cranf1M (28.1%, w/w, dry). Cranf1b was then subjected to separation using a PGC HyperSep Hypercarb SPE cartridge (1 gram,
Thermofisher Scientific, Waltham, MA, USA). The PGC cartridge was firstly conditioned by 50% ACN/water and then equilibrated with 100% d.i. water. Two mL of 20 mg/mL Cranf1b aqueous solution
was then loaded onto the PGC cartridge and eluted sequentially with d.i. water, 10% ACN/H2O/0.1% TFA and 30% ACN/H2O/0.1% TFA. The 10% ACN/H2O/0.1% TFA PGC eluent (cPOS, 15% w/w, dry) was
then separated using a TSKgel Amide-80 HR HILIC column (4.6 × 250 mm, 5 μm, TOSOH Bioscience, Tokyo, Japan) at 1 mL/min and 35 °C. The column was eluted with a linear gradient from 80%
ACN/H2O/0.1% formic acid to 40% ACN/H2O/0.1% formic acid over 30 min. uG3m2 and 1 were collected at 10 min and 12.5 min, respectively (Fig. 2). Trituration of Cranf1b to remove iridoid
terpenes was accomplished by sonicating 200 mg Cranf1b in 30 mL 95% ethanol/water and then centrifuging the solids at 17,000x g for 8 min. The collected solids were then dissolved in d.i.
H2O and lyophilized. The trituration step was repeated twice prior to further separation by PGC chromatography to provide sample cPOS-t (91.7%, w/w dry). URONIC ACID IDENTIFICATION OF CPOS
The composition of the poly-galacturonic acid backbone of cPOS was determined by acid hydrolysis of cPOS followed by derivatization with 1-phenyl-3-methyl-5-pyrazolone (PMP)47. Briefly, 10
mg cPOS was hydrolyzed in 1 mL 2 M TFA at 90 °C for 5 h. After cooling to room temperature, the reaction mixture was centrifuged at 3000 rpm for 5 min. The supernatant was evaporated _in
vacuo_ to remove residual TFA. The hydrolysate mixture was dissolved in 1 mL distilled water and treated with 30 µL NaOH (0.3 M) and 20 µL PMP solution (0.5 M in methanol) for
derivatization. The mixture was incubated at 70 °C for 60 min, cooled to room temperature and neutralized with 30 µL of HCl (0.3 M). The mixture was then extracted with 1 mL chloroform. The
aqueous layer was filtered by passing through a 0.45 µm syringe filter. Glucuronic acid (2.0 mM), galacturonic acid (2.0 mM) and a distilled water blank were derivatized the same as
described above. HPLC analyses of PMP-labeled samples were conducted on a Prominence i-series system (Shimadzu) using a C18 column (4.6 mm × 250 mm, 5 𝜇m, J.T. Baker Inc., Phillipsburg, NJ,
USA). The injection volume was 20 µL with a flow rate of 1.0 mL/min at 35 °C. Mobile phase A was 10% ACN/water with 0.045% KH2 PO4–0.05% triethylamine buffer (pH 7.5) and mobile phase B was
100% ACN. The column was eluted with 6% B for 4 min, 6–12% for 1 min and then 12% B for 20 min. The UV detection wavelength was 254 nm. BACTERIAL STRAINS AND CULTIVATION _E. coli_ CFT073 was
obtained from a cryogenically frozen stock at the laboratory of P.S.C. and stored at −80 °C in a 1:1 mixture of LB broth and 50% glycerol by volume. LB broth and LB agar were used for
routine cultivation. Liquid M9 minimal medium was prepared as described previously16, and M9 minimal medium agar plates were prepared with 1.5% noble agar to avoid impurities present in
bacteriological agar. _IN VITRO_ QUIESCENCE INHIBITION ASSAY The procedure for this assay closely follows a published protocol16. In general, overnight cultures of _E. coli_ CFT073 were
prepared in 0.4% glucose M9 minimal medium as aforementioned. Bacteria from this culture were diluted to a final concentration of 105 CFU in 4 mL of liquid overlay media (0.2% glucose M9
minimal medium with 0.9% noble agar at 45 °C). Each 4 mL overlay inoculum was poured over a pre-warmed (37 °C) 0.2% glucose M9 minimal media agar plate immediately after inoculation. These
plates were allowed to solidify at room temperature with lids slightly ajar. Once solidified, test spots were added to the overlay media and allowed to dry before incubating the plate upside
down at 37 °C for 24 h. Test spots were 10 μL of each test solution at 10 mg/mL in M9 minimal media unless indicated otherwise. Non-growing _E. coli_ in the overlay were considered to be
quiescent if growth could be induced with the positive control (3 co-spots; 5 μL each of tyrosine, lysine, and methionine solutions at 0.1 mg/mL; co-spots added sequentially to the identical
location on the agar). To test the effects in glucose-free conditions, glucose was not added to any of the media components prior to completing the assay. When adding glucose back to the
glucose-free plates, a 10 μL spot of 20% glucose in water (2 mg; sufficient to support bacteria growth) was added directly following and to the identical location as the dried test spot. For
all quiescence assays, a positive result was defined as observable bacteria growth at the site of a test sample following 24 h incubation. A negative result was defined as no observable
bacteria growth at the site of a test sample. Images of agar plates were made after 24 h using a Molecular imager Gel Doc XR+ (Bio-rad, Hercules, CA, USA) system with Image Lab Software.
PERSISTER CELL VIABILITY ASSAY The procedure for this assay closely followed a published protocol16. _E. coli_ CFT073 was streaked from cryogenically frozen stocks onto LB agar plates and
incubated overnight at 37 °C. A loopful of cells from the plate was added to 10 mL 0.4% glucose M9 minimal media in a 125 mL culture flask and incubated overnight at 37 °C and 200 rpm.
Cultures were diluted in fresh 0.2% glucose M9 minimal media supplemented with test samples (1 mg/mL) to an optical density (OD600) of 0.1 (~108 CFU/mL) and grown in the presence of
ampicillin sodium at 10x MIC (0.1 mg/mL) to generate persister fractions. Cultures were grown in the absence of ampicillin and/or cranberry constituents as controls. The cultures were
incubated shaking (200 rpm) at 37 °C and viable counts were measured at 0, 4, and 24 hours by plating on LB media. The persister cell viability assays were completed in triplicate and
analyzed for statistical significance. STATISTICS Persister cell viability assays were compared using a two-tailed student’s _t_ test. _P_-values ≤ 0.05 were considered statistically
significant. REFERENCES * Stamm, W. E. & Norrby, S. R. Urinary tract infections: disease panorama and challenges. _J. Infect. Dis._ 183, S1–4 (2001). Article PubMed Google Scholar *
Flores-Mireles, A. L., Walker, J. N., Caparon, M. & Hultgren, S. J. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. _Nat. Rev. Microbiol._ 13,
269–284 (2015). Article CAS PubMed PubMed Central Google Scholar * Aydin, A., Ahmed, K., Zaman, I., Khan, M. S. & Dasgupta, P. Recurrent urinary tract infections in women. _Int.
Urogynecol. J._ 26, 795–804 (2015). Article PubMed Google Scholar * Foxman, B. Urinary tract infection syndromes: occurrence, recurrence, bacteriology, risk factors, and disease burden.
_Infect. Dis. Clin. North Am._ 28, 1–13 (2014). Article PubMed Google Scholar * Foxman, B. & Brown, P. Epidemiology of urinary tract infections: Transmission and risk factors,
incidence, and costs. _Infect. Dis. Clin. North. Am._ 17, 227–241 (2003). Article PubMed Google Scholar * Hunstad, D. A. & Justice, S. S. Intracellular lifestyles and immune evasion
strategies of uropathogenic _Escherichia coli_. _Annu. Rev. Microbiol._ 64, 203–221 (2010). Article CAS PubMed Google Scholar * Ejrnaes, K. _et al_. Pulsed-field gel electrophoresis
typing of _Escherichia coli_ strains from samples collected before and after pivmecillinam or placebo treatment of uncomplicated community-acquired urinary tract infection in women. _J.
Clin. Microbiol._ 44, 1776–1781 (2006). Article CAS PubMed PubMed Central Google Scholar * Kerrn, M. B., Struve, C., Blom, J., Frimodt-Moller, N. & Krogfelt, K. A. Intracellular
persistence of _Escherichia coli_ in urinary bladders from mecillinam-treated mice. _J. Antimicrob. Chemother._ 55, 383–386 (2005). Article CAS PubMed Google Scholar * Rosen, D. A.,
Hooton, T. M., Stamm, W. E., Humphrey, P. A. & Hultgren, S. J. Detection of intracellular bacterial communities in human urinary tract infection. _PLOS Med._ 4, e329 (2007). Article
PubMed PubMed Central Google Scholar * Silverman, J. A., Schreiber, H. L., Hooton, T. M. & Hultgren, S. J. From physiology to pharmacy: Developments in the pathogenesis and treatment
of recurrent urinary tract infections. _Curr. Urol. Rep._ 14, 448–456 (2013). Article PubMed PubMed Central Google Scholar * Shah, D. _et al_. Persisters: a distinct physiological state
of E. coli. _BMC Microbiol._ 6, 53 (2006). Article PubMed PubMed Central CAS Google Scholar * Lewis, K. Persister cells: molecular mechanisms related to antibiotic tolerance. _Handb.
Exp. Pharmacol_., 121–133, (2012). * Lewis, K. Persister cells. _Annu. Rev. Microbiol._ 64, 357–372 (2010). Article CAS PubMed Google Scholar * Lewis, K. Multidrug tolerance of biofilms
and persister cells. _Curr. Top. Microbiol. Immunol._ 322, 107–131 (2008). CAS PubMed Google Scholar * Joers, A., Kaldalu, N. & Tenson, T. The frequency of persisters in Escherichia
coli reflects the kinetics of awakening from dormancy. _J. Bacteriol._ 192, 3379–3384 (2010). Article CAS PubMed PubMed Central Google Scholar * Leatham-Jensen, M. P. _et al_.
Uropathogenic Escherichia coli metabolite-dependent quiescence and persistence may explain antibiotic tolerance during urinary tract infection. _mSphere_ 1, e00055–00015 (2016). Article CAS
PubMed PubMed Central Google Scholar * Putrinš, M. _et al_. Phenotypic heterogeneity enables uropathogenic _Escherichia coli_ to evade killing by antibiotics and serum complement.
_Infect. Immun._ 83, 1056–1067 (2015). Article PubMed PubMed Central CAS Google Scholar * Niu, H. _et al_. Identification of anti-persister activity against uropathogenic Escherichia
coli from a clinical drug library. _Antibiotics_ 4, 179–187 (2015). Article CAS PubMed PubMed Central Google Scholar * Orman, M. A. & Brynildsen, M. P. Inhibition of stationary
phase respiration impairs persister formation in _E. coli_. _Nat. Commun._ 6, 7983 (2015). Article ADS CAS PubMed PubMed Central Google Scholar * Kim, J. S. _et al_. Fumarate-mediated
persistence of _Escherichia coli_ against antibiotics. _Antimicrob. Agents. Chemother._ 60, 2232–2240 (2016). Article CAS PubMed PubMed Central Google Scholar * Vasileiou, I.,
Katsargyris, A., Theocharis, S. & Giaginis, C. Current clinical status on the preventive effects of cranberry consumption against urinary tract infections. _Nutr. Res._ 33, 595–607
(2013). Article CAS PubMed Google Scholar * Jepson, R. G., Williams, G. & Craig, J. C. Cranberries for preventing urinary tract infections. _Cochrane Database Syst. Rev._ 10,
Cd001321 (2012). PubMed Google Scholar * Sobota, A. E. Inhibition of bacterial adherence by cranberry juice: potential use for the treatment of urinary tract infections. _J. Urol._ 131,
1013–1016 (1984). Article CAS PubMed Google Scholar * Gupta, K. _et al_. Cranberry products inhibit adherence of p-fimbriated _Escherichia coli_ to primary cultured bladder and vaginal
epithelial cells. _J. Urol._ 177, 2357–2360 (2007). Article CAS PubMed PubMed Central Google Scholar * Caljouw, M. A. _et al_. Effectiveness of cranberry capsules to prevent urinary
tract infections in vulnerable older persons: a double-blind randomized placebo-controlled trial in long-term care facilities. _J. Am. Geriatr. Soc._ 62, 103–110 (2014). Article PubMed
Google Scholar * Maisuria, V. B., Los Santos, Y. L.-d, Tufenkji, N. & Déziel, E. Cranberry-derived proanthocyanidins impair virulence and inhibit quorum sensing of _Pseudomonas
aeruginosa_. _Sci. Rep._ 6, 30169 (2016). Article ADS CAS PubMed PubMed Central Google Scholar * Howell, A. B. _et al_. A-type cranberry proanthocyanidins and uropathogenic bacterial
anti-adhesion activity. _Phytochemistry_ 66, 2281–2291 (2005). Article CAS PubMed Google Scholar * Zafriri, D., Ofek, I., Adar, R., Pocino, M. & Sharon, N. Inhibitory activity of
cranberry juice on adherence of type 1 and type P fimbriated _Escherichia coli_ to eucaryotic cells. _Antimicrob. Agents Chemother._ 33, 92–98 (1989). Article CAS PubMed PubMed Central
Google Scholar * Jensen, H. D., Struve, C., Christensen, S. B. & Krogfelt, K. A. Cranberry juice and combinations of its organic acids are effective against experimental urinary tract
infection. _Front. Microbiol._ 8, 542 (2017). PubMed PubMed Central Google Scholar * Sun, J. _et al_. Cranberry (_Vaccinium macrocarpon_) oligosaccharides decrease biofilm formation by
uropathogenic Escherichia coli. _J. Funct. Foods_ 17, 235–242 (2015). Article CAS PubMed PubMed Central Google Scholar * Coleman, C. M. _et al_. Arabinoxyloglucan oligosaccharides may
contribute to the antiadhesive properties of porcine urine after cranberry consumption. _J. Nat. Prod._ 82, 589–605 (2019). Article CAS PubMed Google Scholar * Hotchkiss, A. T. Jr. _et
al_. Cranberry xyloglucan structure and inhibition of _Escherichia coli_ adhesion to epithelial cells. _J. Agric. Food Chem._ 63, 5622–5633 (2015). Article CAS PubMed Google Scholar *
Spiro, M. D. _et al_. Purification and characterization of biologically active 1, 4-linked α-d-oligogalacturonides after partial digestion of polygalacturonic acid with
endopolygalacturonase. _Carbohydr. Res._ 247, 9–20 (1993). Article CAS Google Scholar * Cipriani, T. R. _et al_. Polygalacturonic acid: Another anti-ulcer polysaccharide from the
medicinal plant _Maytenus ilicifolia_. _Carbohydr. Polym._ 78, 361–363 (2009). Article CAS Google Scholar * Marcon, M. V., Carneiro, P. I. B., Wosiacki, G., Beleski-Carneiro, E. &
Petkowicz, C. L. O. Pectins from apple pomace–characterization by 13C and 1H NMR spectroscopy. _Ann. Magn. Reson._ 4, 56–63 (2005). Google Scholar * Cheng, H. N. & Neiss, T. G. Solution
NMR spectroscopy of food polysaccharides. _Polymer Reviews_ 52, 81–114 (2012). Article CAS Google Scholar * Jensen, H. D., Krogfelt, K. A., Cornett, C., Hansen, S. H. & Christensen,
S. B. Hydrophilic carboxylic acids and iridoid glycosides in the juice of American and European cranberries (_Vaccinium macrocarpon_ and _V. oxycoccos_), lingonberries (_V. vitisidaea_), and
blueberries (_V. myrtillus_). _J. Agric. Food Chem._ 50, 6871–6874 (2002). Article CAS PubMed Google Scholar * Dinda, B., Debnath, S. & Harigaya, Y. Naturally occurring iridoids. A
review, part 1. _Chem. Pharm. Bull._ 55, 159–222 (2007). Article CAS Google Scholar * Chaudhuri, R. K., Afifi-Yazar, F. Ü., Sticher, O. & Winkler, T. 13C NMR spectroscopy of naturally
occurring iridoid glucosides and their acylated derivatives. _Tetrahedron_ 36, 2317–2326 (1980). Article CAS Google Scholar * Demi̇rezer, L. Ö., Gürbüz, F., Güvenalp, Z., STRÖCH, K.
& Zeeck, A. Iridoids, flavonoids and monoterpene glycosides from _Galium verum_ subsp_. verum_. _Turk. J. Chem._ 30, 525–534 (2006). Google Scholar * Croxall, G. _et al_. Molecular
epidemiology of extraintestinal pathogenic _Escherichia coli_ isolates from a regional cohort of elderly patients highlights the prevalence of ST131 strains with increased antimicrobial
resistance in both community and hospital care settings. _J Antimicrob Chemother_ 66, 2501–2508 (2011). Article CAS PubMed Google Scholar * Gibreel, T. M. _et al_. Population structure,
virulence potential and antibiotic susceptibility of uropathogenic _Escherichia coli_ from Northwest England. _J Antimicrob Chemother_ 67, 346–356 (2012). Article CAS PubMed Google
Scholar * Pedrolli, D. B., Monteiro, A. C., Gomes, E. & Carmona, E. C. Pectin and pectinases: production, characterization and industrial application of microbial pectinolytic enzymes.
_Open Biotechnol. J._ 3, 9–18 (2009). Article CAS Google Scholar * Hasegawa, S. & Nagel, C. W. The characterization of an α, β-unsaturated digalacturonic acid. _J. Biol. Chem._ 237,
619–621 (1962). CAS PubMed Google Scholar * Linhardt, R., Galliher, P. & Cooney, C. Polysaccharide lyases. _Applied Biochem. Biotechnol._ 12, 135–176 (1987). Article Google Scholar
* van Alebeek, G. J., Christensen, T. M., Schols, H. A., Mikkelsen, J. D. & Voragen, A. G. Mode of action of pectin lyase A of _Aspergillus niger_ on differently C(6)-substituted
oligogalacturonides. _J. Biol. Chem._ 277, 25929–25936 (2002). Article PubMed CAS Google Scholar * Ruhaak, L. R. _et al_. Glycan labeling strategies and their use in identification and
quantification. _Anal. Bioanal. Chem._ 397, 3457–3481 (2010). Article CAS PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS This work was supported, in part, by
Ocean Spray Cranberries, Inc. (Lakeville-Middleboro, MA, USA). RD was financially supported by the Omar Magnate Foundation Fellowship. Research reported in this publication was made possible
by the use of equipment and services available through the RI-INBRE Centralized Research Core Facility, which is supported by the Institutional Development Award (IDeA) Network for
Biomedical Research Excellence from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM103430, and by the National Science
Foundation EPSCoR Cooperative Agreement #EPS-1004057. AUTHOR INFORMATION Author notes * These authors contributed equally: Jiadong Sun and Robert W. Deering. AUTHORS AND AFFILIATIONS *
Department of Biomedical and Pharmaceutical Sciences, College of Pharmacy, University of Rhode Island, Kingston, RI, 02881, USA Jiadong Sun, Robert W. Deering, Zhiyuan Peng, Laila Najia,
Navindra P. Seeram & David C. Rowley * Laboratory of Bioorganic Chemistry, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD,
20814, USA Jiadong Sun * Ocean Spray Cranberries, Inc., One Ocean Spray Drive, Lakeville-Middleboro, MA, 02349, USA Christina Khoo * Department of Cell and Molecular Biology, University of
Rhode Island, Kingston, RI, 02881, USA Paul S. Cohen Authors * Jiadong Sun View author publications You can also search for this author inPubMed Google Scholar * Robert W. Deering View
author publications You can also search for this author inPubMed Google Scholar * Zhiyuan Peng View author publications You can also search for this author inPubMed Google Scholar * Laila
Najia View author publications You can also search for this author inPubMed Google Scholar * Christina Khoo View author publications You can also search for this author inPubMed Google
Scholar * Paul S. Cohen View author publications You can also search for this author inPubMed Google Scholar * Navindra P. Seeram View author publications You can also search for this author
inPubMed Google Scholar * David C. Rowley View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS D.C.R., J.S., R.W.D. and P.S.C. conceived and
designed the experiments. J.S. and R.W.D. prepared the manuscript and contributed equally to this work. J.S. and R.W.D. performed chemical purification and characterization of cranberry
materials. R.W.D and L.N. performed _in vitro_ quiescence and persister cell assays. Z.P. contributed to Figures 2 and S3. L.N. contributed to Figure 8. C.K., P.S.C., N.P.S and D.C.R edited
and approved the final version of the manuscript. CORRESPONDING AUTHOR Correspondence to David C. Rowley. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests.
ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION
SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation,
distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and
indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to
the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will
need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE
CITE THIS ARTICLE Sun, J., Deering, R.W., Peng, Z. _et al._ Pectic Oligosaccharides from Cranberry Prevent Quiescence and Persistence in the Uropathogenic _Escherichia coli_ CFT073. _Sci
Rep_ 9, 19590 (2019). https://doi.org/10.1038/s41598-019-56005-w Download citation * Received: 06 March 2019 * Accepted: 04 December 2019 * Published: 20 December 2019 * DOI:
https://doi.org/10.1038/s41598-019-56005-w SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative