An intra-bacterial activity for a t3ss effector
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Many Gram-negative bacterial pathogens interact with mammalian cells by using type III secretion systems (T3SS) to inject virulence proteins into host cells. A subset of these
injected protein ‘effectors’ are enzymes that inhibit the function of host proteins by catalyzing the addition of unusual post-translational modifications. The _E. coli_ and _Citrobacter
rodentium_ NleB effectors, as well as the _Salmonella enterica_ SseK effectors are glycosyltransferases that modify host protein substrates with N-acetyl glucosamine (GlcNAc) on arginine
residues. This post-translational modification disrupts the normal functioning of host immune response proteins. T3SS effectors are thought to be inactive within the bacterium and fold into
their active conformations after they are injected, due to the activity of chaperones that keep the effectors in a structural state permissive for secretion. While performing mass
spectrometry experiments to identify glycosylation substrates of NleB orthologs, we unexpectedly observed that the bacterial glutathione synthetase (GshB) is glycosylated by NleB on arginine
residue R256. NleB-mediated glycosylation of GshB resulted in enhanced GshB activity, leading to an increase in glutathione production, and promoted _C. rodentium_ survival in oxidative
stress conditions. These data represent, to our knowledge, the first intra-bacterial activity for a T3SS effector and show that arginine-GlcNAcylation, once thought to be restricted to host
cell compartments, also plays an important role in regulating bacterial physiology. SIMILAR CONTENT BEING VIEWED BY OTHERS ARGININE GLYCOSYLATION ENHANCES METHYLGLYOXAL DETOXIFICATION
Article Open access 15 February 2021 CATALYTIC DXD MOTIF CAGED IN ASX-TURN AND MET–AROMATIC INTERACTION ATTENUATES THE PATHOGENIC GLYCOSYLATION OF SSEK2/NLEB2 EFFECTORS Article Open access
11 November 2022 QUANTITATIVE PROTEOMIC SCREEN IDENTIFIES ANNEXIN A2 AS A HOST TARGET FOR _SALMONELLA_ PATHOGENICITY ISLAND-2 EFFECTORS SOPD2 AND PIPB2 Article Open access 08 December 2021
INTRODUCTION The NleB (_Escherichia coli_ and _Citrobacter rodentium_) and SseK (_Salmonella enterica_) enzymes are type three secretion system (T3SS) effector proteins that glycosylate host
proteins with N-acetyl glucosamine (GlcNAc) on arginine residues to subvert their function in the innate immune system1,2,3. Arginine glycosylation is unusual because it occurs on the
guanidinium groups of arginines, which are poor nucleophiles. The NleB/SseK orthologs share high degrees of structural similarity and consist of a catalytic domain including essential DXD
and HEN motifs, a helix-loop-helix (HLH) domain, and a C-terminal lid domain4,5,6. Several ‘death domain’-containing proteins such as the Fas-Associated protein with Death Domain (FADD),
tumor necrosis factor receptor type 1-associated death domain protein (TRADD), and the receptor interaction serine/threonine-protein kinase 1 (RIPK1) are NleB/SseK substrates2. These
effectors disrupt tumor necrosis factor receptor (TNFR)-associated factor (TRAF) signaling, leading to inhibition of the pro-inflammatory NF-κB pathway1,2,3. T3SS effectors are chaperoned in
the bacterium after their synthesis to keep them partially unfolded and competent for secretion, as well as for targeting the effectors to the T3SS sorting platform7. The chaperones are
then stripped from their effector substrates at the sorting platform and the effectors are secreted in an unfolded conformation8. T3SS effectors are generally believed to be inactive until
they are injected into host cells, where they then fold into their active conformations9. Some precedent for type IV secretion system (T4SS) effector activity in both the bacterium and the
host may exist in plant pathogens. _Agrobacterium tumefaciens_ transfers a nucleoprotein complex into plant cells. The VirD2 protein is associated with the transferred DNA (T-DNA). VirD2 has
endonuclease activity within the bacterium to initiate T-DNA transfer10. VirD2 also targets the nucleoprotein complex in the plant cell nucleus, where it assists in integrating T-DNA into
plant chromosomes11. Therefore, VirD2 may have enzymatic functions both within the bacterium and in the host plant cell. A recent study also indicated that _Yersinia pseudotuberculosis_ uses
the secreted T3SS translocator YopD to control RNA regulators and increase the abundance of LcrF, a common transcriptional activator of other T3SS effector genes12. N-linked protein
glycosylation on arginine has also been reported for the EarP glycosyltransferase from _E. coli_ and _Pseudomonas aeruginosa_13. In this case, a single arginine rhamnosylation event
activates the function of the polyproline-specific bacterial translation elongation factor EF-P13. Although the relatively inert guanidine group of arginine was previously thought to impede
nucleophilic attack onto donor substrates, it is now clear that many bacterial enzymes have overcome this barrier. The enzymology and functional roles of arginine glycosylation, methylation,
phosphorylation, and ADP-ribosylation were recently reviewed14. While characterizing additional NleB glycosylation substrates, we made the unexpected observation that the bacterial
glutathione synthetase (GshB) is glycosylated by NleB on an arginine residue. This glycosylation contributes to bacterial survival in hydrogen peroxide stress conditions by enhancing GshB
activity and increasing intracellular levels of glutathione (GSH). Thus, NleB is active and performs important biological functions within the bacterium, prior to its secretion. RESULTS NLEB
GLYCOSYLATES GSHB We performed mass spectrometry experiments to identify new glycosylation substrates of NleB orthologs from EHEC strains associated with human disease outbreaks. These
experiments were conducted by infecting HEK293T cells with EHEC strains that express NleB1. We then used an anti-Arg-R-GlcNAc monoclonal antibody15 for the antibody-based capture of
arginine-GlcNAcylated peptides to perform proteome-wide assessment of Arg-GlcNAcylation mediated by NleB1, as described previously16. We unexpectedly observed glycosylation of _E. coli_
glutathione synthetase (GshB) on arginine residue R256 as the most abundant Arg-GlcNAcylated peptide enriched from samples, followed by the known human NleB1 target FADD16 (Supp. Table 1).
Because _C. rodentium_ encodes only one copy of NleB, while most EHEC strains encode two copies (NleB1 and NleB2), we subsequently attempted to reproduce our initial findings using _C.
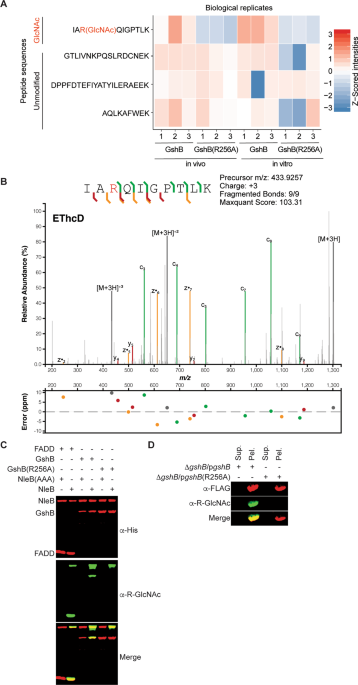
rodentium_ NleB and GshB using _in vivo_4 or _in vitro_17 studies to investigate glycosylation within this protein. Using these assays, we observed that GshB is exclusively modified on R256,
with this glycosylation event absent in GshB(256 A) expressing strains [Fig. 1A; -log10(p-value) of 1.77 and 2.78 from _in vivo_ and _in vitro_ assays respectively, Supp. Table 2]. To
further test the localization of the glycosylation, _in vivo_ glycosylated GshB was subjected to Electron-Transfer/Higher-Energy Collision Dissociation (EThcD) fragmentation, confirming the
attachment of the GlcNAc residue to R256 (Fig. 1B). These data support the notion that the glutathione synthetase GshB from the attaching/effacing pathogens EHEC and _C. rodentium_ is
glycosylated at R256 under _in vivo_ conditions. To corroborate our mass spectrometry data, we conducted _in vitro_ glycosylation assays17 with the Anti-R-GlcNAc monoclonal antibody15 by
expressing recombinant forms of wild-type (WT) GshB or GshB(256 A). NleB glycosylated the WT, but not the R256A GshB mutant (Fig. 1C), consistent with our _in vivo_ and _in vitro_ MS assays.
Within these assays, FADD was used as a positive control as a known NleB substrate16. The NleB(AAA) mutant, which lacks glycosylation activity1, was used as a negative control. We then
generated a _gshB_ deletion in _C. rodentium_ and complemented this mutant with FLAG-tagged versions of either WT or GshB(R256A). Both FLAG-tagged forms of GshB were isolated from _C.
rodentium_; only WT GshB was glycosylated by the endogenous levels of NleB (Fig. 1D). Neither secretion of GshB nor its glycosylation in the culture medium by NleB was observed, suggesting
that NleB glycosylation of GshB occurred within the bacterium. NLEB PROMOTES OXIDATIVE STRESS RESISTANCE Glutathione (GSH) is generated in Gram-negative bacteria in a two-step process by
γ-glutamylcysteine synthetase (_gshA_) and glutathione synthetase (_gshB_). GshA uses glutamate and cysteine as substrates to catalyze the formation of γ-L-glutamylcysteine. GshB catalyzes
GSH production by ligating glycine and γ-L-glutamylcysteine18. _Salmonella_ lacking either enzyme do not produce GSH and exhibit increased susceptibility to oxidative stress19. A _Salmonella
gshA_ deletion is hypersensitive to H2O2, and _gshA_ and _gshB_ deletions are hypersensitive to both nitric oxide (NO) and S-nitrosoglutathione (GSNO)19. Similarly, in _Pseudomonas
aeruginosa_, _gshA_ and/or _gshB_ deletions are more sensitive to environmental stress and attenuated for virulence20. In _Streptococcus pneumoniae_, mutating _gshT_, an ABC transporter
required for GSH import, increased _S. pneumoniae_ sensitivity to superoxide, and was attenuated in a mouse model of infection21. Deleting _gshA_ and _gshB_ from _P. aeruginosa_ attenuates
several virulence-associated phenotypes including motility and biofilm formation. GSH was also shown to activate both the _P. aeruginosa_ T3SS and a subset of T6SS genes22. Glutathione
binding to the _Listeria monocytogenes_ master regulator PrfA is also critical to the virulence of this intracellular pathogen23. To determine whether GshB glycosylation by NleB has
functional significance in promoting resistance to oxidative stress, we compared the growth rates of bacterial strains in the presence or absence of H2O2. Both the _nleB_ and _gshB_ mutants
had a significant growth defect in the presence of H2O2 (Fig. 2A), despite having similar growth rates in the absence of H2O2 (Fig. 2B). The _nleB_ mutant phenotype was complemented by
expressing WT _nleB_ on a plasmid, but not by expressing the inactive _nleB_(AAA) mutant. Mutating the GshB R256 residue to alanine (R256A) had no impact on bacterial growth rates. These
data highlight a new role for NleB, namely its requirement for _C. rodentium_ survival in peroxide stress conditions. ARG-GLYCOSYLATION ENHANCES GSHB ACTIVITY We then tested the hypothesis
that GshB glycosylation enhances GshB-mediated production of glutathione (GSH), consistent with the bacterial growth phenotypes in the presence of H2O2. We incubated _C. rodentium_ lysates
prepared from cultures grown in the presence or absence of 2.4 mM H2O2 with glutathione _S_-transferase (GST) and 1-chloro-2,4-dinitrobenzene (CDNB) to quantify GSH concentrations in
bacterial lysates24. The WT strain produced significantly more GSH (3.4 + 0.9 pmoles/s) than did the _nleB_ mutant (2.3 + 0.6 pmoles/s), irrespective of the presence of H2O2 (Fig. 3A,B). To
verify that the growth defect of the _nleB_-deleted strain in H2O2 was due to insufficient GSH, we supplemented bacterial cultures with 5.0 mM GSH and observed partial restoration of the
growth of the _nleB_ mutant in the presence of H2O2 (Fig. 3C). We then reconstituted an _in vitro_ GSH production assay using purified, recombinant GshA, GST, as well as GshB that was
purified from _E. coli_ BL21(DE3), following its co-expression with either NleB or NleB(AAA) (Fig. 4A). Thus, we aimed to generate either glycosylated GshB (when co-expressed with WT NleB)
or unglycosylated GshB [when mutated to R256A and/or when WT GshB was co-expressed with NleB(AAA)] to directly characterize the impact of GshB glycosylation on GshB activity. Glycosylation
of GshB on R256 in this assay was confirmed using western blotting, showing that NleB was active when expressed in _E. coli_ BL21(DE3) (Fig. 4B). The GshB enzyme purified from the WT NleB
co-expression strain was significantly more active than the GshB enzyme purified from the NleB(AAA) co-expression strain (1.5 + 0.1 vs. 1.0. + 0.1 pmoles/s) (Fig. 4C). NleB had no impact on
GSH production catalyzed by GshB(R256A). We obtained similar data when GshB was first purified from _E. coli_ and then subsequently glycosylated by NleB _in vitro_ (Fig. S1). We also
considered whether _Salmonella_ might glycosylate GshB _in vivo_. We did not observe such glycosylation under conditions performed similarly to those using _C. rodentium_ (Fig. S2A).
However, _Salmonella_ is significantly more resistant to H2O2 than is _C. rodentium_, due to the presence of redundant catalases and alkyl hydroperoxide reductases25,26,27. Consistent with
this, we failed to observe a growth phenotype when we subjected WT _Salmonella_ and all possible combinations of _sseK1/K2/K3_ mutants to 12.0 mM H2O2, a concentration 5-times greater than
that used for _C. rodentium_ experiments (Fig. S2B). These data suggest that the SseK enzymes are not significantly involved in H2O2 resistance by _Salmonella_. However, we further
considered the possibility that some degree of GshB glycosylation might be observed _in vitro_ using purified recombinant proteins. We especially considered this because of previous findings
demonstrating that NleB/SseK substrate specificity may be more broad _in vitro_ as compared to _in vivo_ assays, particularly when the enzyme is present at supraphysiological
concentration16. We incubated GshB with all known NleB/SseK orthologs from EHEC, EPEC, _C. rodentium_, and _Salmonella_ and observed that EHEC NleB1, EPEC NleB1, and _Salmonella_ SseK1 all
glycosylated GshB, whereas NleB2, SseK2, and SseK3 did not (Fig. S2C). Whether GshB glycosylation by EHEC and/or EPEC NleB1 may affect oxidative stress resistance in these attaching/effacing
pathogens awaits further experimentation. Although no chaperone for NleB has been identified, we also considered whether the presence of CesT, a multi-cargo chaperone that interacts with at
least 9 other effectors28, might affect NleB activity. We especially considered this possibility because effector-binding and secretion activities are separable for CesT29. However, we did
not observe any impact on NleB activity in a _C. rodentium cesT_ mutant (Fig. S2A), nor did we observe an interaction between NleB and CesT, as assessed using affinity chromatography (_data
not shown_). Thus, NleB activity appears to be independent of CesT. DISCUSSION NleB is an arginine glycosyltransferase that modifies several host proteins, leading to inactivation of the
host pro-inflammatory response mediated by NF-κB4,6. Here we discovered that NleB also plays a significant role in bacterial survival in oxidative stress conditions by glycosylating GshB to
enhance GSH production. In this case, rather than inactivating the NleB substrates, as is seen with the mammalian targets, Arg-GlcNAcylation significantly activates the bacterial GshB
enzyme. It remains to be determined why GshB glycosylation enhances its enzyme activity. The glycosylated R256 is distant from the active site (Fig. S2B), as inferred from the _E. coli_ GshB
structure [PDB 1GSA;30]. Possible mechanisms could include changes in GshB oligomerization state, a conformational change of the GshB active site, or enhanced affinity for/localization with
GshA. GshB is an abundant cytoplasmic protein31. In our assays, we observed a 50–100% increase in GshB activity (Figs. 3–4), despite only a relatively low degree of overall GshB
Arg-GlcNAcylation (occupancy of ~5%, Supp. Table 1). It is therefore possible that Arg-GlcNAcylation may increase GshB activity by as much as 10-fold. Formal testing of this hypothesis
awaits the complete separation of glycosylated from unglycosylated forms of GshB. NleB/SseK activities in the host cell have been characterized for their inhibition of proteins involved in
the innate immune response1,2,3,4,6. Previous work with Ear-P showed that Arg-rhamnosylation activates EF-P in the bacterial cytosol13, to avoid ribosome stalling during the synthesis of
poly-proline regions32. It is now clear from our work that Arg-GlcNAcylation also provides an important activating function, namely the NleB-mediated activation of GshB through R256
glycosylation. The abundance and functional significance of cytoplasmic bacterial glycoproteins may be a fruitful area for future studies. Regardless of the specific activating mechanism,
these data represent, to our knowledge, the first example of a T3SS effector functioning within both the bacterium and within the host cell. It is possible that other _C. rodentium_ proteins
are glycosylated by NleB to provide the organism a means by which to integrate T3SS activation and effector synthesis with bacterial physiological processes such as transcriptional
regulation, flagellar biosynthesis, and quorum sensing. It is also possible that other T3SS effectors are substrates of NleB, and that other T3SS effectors with enzymatic activities are
active within the bacterium, a concept similar to the role of metaeffectors playing important regulatory roles in _Legionella pneumophila_ biology33. The extent to which other effectors with
enzymatic activities may be active within the bacterium may emerge as a new area of investigation. MATERIALS AND METHODS STRAINS AND MOLECULAR CLONING The plasmids and strains used in this
study are listed in Table 1. WT _nleB_ (_C. rodentium_) and its derivative DAD221–223/AAA were cloned into pET42a. FADD was cloned into pET15a. WT _gshB_ and its derivative _gshB_ R256A, as
well as _gshA_, were cloned in pET28a using ABC cloning34. The _C. rodentium gshB_ deletion was generated using lambda red recombination35. PROTEIN PURIFICATION Proteins were expressed from
_E. coli_ BL21 (DE3) and induced with 0.5 mM IPTG when cultures reached an OD600 of 0.4. Cells were grown for an additional 4 h at 37 °C and then harvested using centrifugation. Cell pellets
were resuspended in 1/40th culture volume of 50 mM NaH2PO4 pH 8.0 supplemented with 0.5 mg/ml lysozyme and protease inhibitor cocktails (Thermo Scientific). Bacterial suspensions were
incubated on ice for 30 min with occasional shaking, at which time an equal volume 50 mM NaH2PO4 pH 8.0, 2 M NaCl, 8 mM imidazole, 20% glycerol, 2% Triton X-100 was added for additional
incubation on ice for 30 min. Cell lysates were sonicated, centrifuged, and combined with 2 ml Ni-NTA slurry (Qiagen) for 1 h at 4 °C with gentle rotation. The mixture was loaded on a
Poly-Prep Chromatography Column (Bio-Rad) and washed in 50 mM NaH2PO4 pH 8.0, 600 mM NaCl, 60 mM imidazole, 10% glycerol). Proteins were eluted in 50 mM NaH2PO4 pH 8.0, 600 mM NaCl, 250 mM
imidazole, 10% glycerol and then dialyzed into the same buffer lacking imidazole17. ENRICHMENT OF ARGININE-GLYCOSYLATED PEPTIDES FROM CELL LYSATES HEK293T cells were grown in DMEM to 80%
confluency and then infected with EHEC O111:NM R82F236 at a multiplicity of infection of 1.0 for 16 h. Infected cells were washed three times in ice-cold PBS and lysed by scraping with
ice-cold guanidinium chloride lysis buffer (6 M GdmCl, 100 mM Tris pH 8.5, 10 mM TCEP, 40 mM 2-Chloroacetamide) on a bed of ice. Lysates were collected and boiled at 95 °C for 10 minutes
with shaking at 2,000 rpm to shear DNA and inactivate protease activity. Lysates were then cooled for 10 minutes on ice and then boiled again at 95 °C for 10 minutes with shaking at 2,000
rpm. Protein samples (2 mg) were acetone precipitated and dried protein pellets were resuspended in 6 M urea, 2 M thiourea, 40 mM NH4HCO3 and reduced/alkylated prior to digestion with Lys-C
(1/200 w/w) and then with trypsin (1/50 w/w) overnight as previously described37. Digested samples were acidified to a final concentration of 0.5% formic acid and desalted with 50 mg tC18
SEP-PAK (Waters Corporation). tC18 SEP-PAKs were conditioned with buffer B (80% ACN, 0.1% formic acid), washed with 10 volumes of Buffer A* (0.1% TFA, 2% ACN), sample loaded, column washed
with 10 volumes of Buffer A* and bound peptides eluted with buffer B then dried. Arg-GlcNAc peptide affinity purification was performed as previously described16. Protein A/G plus Agarose
beads (Santa Cruz, Santa Cruz CA) were washed with immunoprecipitation buffer (IAP, 10 mM Na2HPO4, 50 mm NaCl, 50 mM MOPS, pH 7.2) and rotated overnight with 10 μg of anti-Arg-GlcNAc
antibody (ab195033, Abcam) at 4 °C. Coupled anti-Arg-GlcNAc beads were then washed with 100 mM sodium borate (pH 9) to remove non-bound proteins and cross-linked for 30 minutes of rotation
using 20 mM dimethyl pimelimidate in 100 mM HEPES, pH 8.0. Cross-linking was quenched by washing beads three times with 200 mM ethanolamine, pH 8.0 and then rotating the beads in an
additional 1 ml of 200 mM ethanolamine, pH 8.0 for 2 hours at 4 °C. Purified peptides were resuspended in 1 ml IAP buffer and peptide lysates were then added to the prepared cross-linked
anti-Arg-GlcNAc antibody beads and rotated for 3 hours at 4 °C. Antibody beads were centrifuged at 3,000 g for 2 minutes at 4 °C and the unbound peptide lysates collected. Antibody beads
were then washed with ice-cold IAP buffer and Arg-GlcNAc peptides eluted using two rounds of acid elution. For each elution round, 100 μl of 0.2% TFA was added and antibody beads allowed to
stand at room temperature with gentle shaking every minute for 10 minutes. Peptide supernatants were collected and desalted using C18 stage tips38 before being dried down and stored until
LC-MS analysis. SP3 ON-BEAD LYS-C DIGESTION OF PURIFIED GSHB Purified tagged GshB was cleaned up using SP3 based purification according to previous protocols39. Samples were first denatured
and reduced using 1% SDS, 10 mM DTT, 100 mM HEPES by boiling at 95 °C, 1,000 rpm for 10 minutes. Samples were then cooled and alkylated with 40 mM 2-chloroacetamide (CAA) for 1 hour at RT in
the dark. The alkylation reactions were then quenched with 40 mM DTT for 10 minutes and then samples precipitated on to SeraMag Speed Beads (GE Healthcare, USA) with ethanol (final
concentration 50% v/v). Samples were shaken for 10 minutes to allow complete precipitation onto beads and then washed three times with 80% ethanol. The beads were resuspended in 100 mM
ammonium bicarbonate containing 1 μg lys-C 1/50 (w/w) and digested overnight at 37 °C. Samples were centrifuged at 14,000 g for 5 minutes to pellet the beads and the supernatant collected
and desalted using C18 stage tips before being dried for LC-MS analysis. IDENTIFICATION OF ARGININE-GLYCOSYLATED AFFINITY ENRICHED PEPTIDES AND HIS-TAGGED PROTEINS USING REVERSED PHASE LC-MS
Purified peptides were resuspended in Buffer A* and separated using a two-column chromatography setup composed of a PepMap100 C18 20 mm × 75 μm trap and a PepMap C18 500 mm × 75 μm
analytical column (Thermo Fisher Scientific)16. Samples were concentrated onto the trap column at 5 μl/min for 5 minutes and infused into either an Orbitrap Fusion Lumos Tribrid Mass
Spectrometer (Thermo Fisher Scientific) for the analysis of enriched Arg-GlcNAc peptides and PRM analysis of Arg-GlcNAcylated GshB or an Orbitrap Elite (Thermo Fisher Scientific) for the
comparison of Arg-GlcNAcylation levels within purified GshB. Gradients (120 minutes) were run by altering the buffer composition from 1% buffer B to 28% B over 90 minutes, then from 28% B to
40% B over 10 minutes, then from 40% B to 100% B over 2 minutes, the composition was held at 100% B for 3 minutes, and then dropped to 3% B over 5 minutes and held at 3% B for another 10
minutes for enriched Arg-GlcNAc modified peptides and comparison of Arg-GlcNAcylation levels within purified GshB. For 120-minute gradients, the Lumos and Elite Mass Spectrometers were
operated in a data-dependent mode automatically switching between the acquisition of a single Orbitrap MS scan HCD or CID fragmentation. For parallel reaction monitoring (PRM) experiments,
the known Lys-C Arg-modified peptide of GshB (IARQIGPTLKEK) was monitored using EThcD fragmentation targeting the predicted m/z for the +2 (m/z 650.2835) and +3 charge (m/z 488.9248) states
over a 95 minute gradient, altering the buffer composition from 1% buffer B to 28% B over 60 minutes, then from 28% B to 40% B over 10 minutes, then from 40% B to 100% B over 2 minutes, held
at 100% B for 3 minutes, and then dropped to 3% B over 5 minutes and held at 3% B for another 15 minutes. MASS SPECTROMETRY DATA ANALYSIS Identification of Arg-glycosylated peptides was
accomplished using MaxQuant (v1.5.3.30)40. Searches were performed against _E. coli_ O127:H6 strain E2348/69 (Uniprot proteome id UP000008205- _E. coli_ O127:H6 strain E2348/69/EPEC,
downloaded 28-07-2014, 4,595 entries) or _C. rodentium_ ICC168 (Uniprot proteome id UP000001889- _C. rodentium_ strain ICC168 downloaded 12/12/2016) proteomes depending on the samples, with
carbamidomethylation of cysteine set as a fixed modification. Searches were performed with Trypsin or Lys-C cleavage specificity depending on the experiment. Two miscleavage events were
allowed, as well as the variable modifications of oxidation of methionine, N-Acetylhexosamine addition to arginine (Arg-GlcNAc) and acetylation of protein N-termini. Precursor mass tolerance
was set to 20 parts-per-million (ppm) for the first search and 10 ppm for the main search, with a maximum false discovery rate (FDR) of 1.0% set for protein and peptide identifications. The
Match Between Runs option was enabled with a precursor match window set to 2 minutes and an alignment window of 10 minutes. For label-free quantitation, the MaxLFQ option within Maxquant41
was enabled, in addition to the re-quantification module. Peptide outputs were processed using the Perseus (v1.4.0.6)42 analysis environment to remove reverse matches and common protein
contaminants with missing values imputed and the peptide intensities z-scored. MS/MS annotations were undertaken using the Interactive Peptide Spectral Annotator43. Mass spectrometry
proteomics data were deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD015752. GLYCOSYLATION ASSAYS _In vitro_ glycosylation assays
were performed as described previously17 using 200 nM of NleB1 or its orthologs with 1 μM of either WT GshB or GshB R256A in 50 mM Tris-HCl pH 7.4, 1 mM UDP-GlcNAc, 10 mM MnCl2, and 1 mM
DTT. After 2 h incubation at RT, samples were subjected to western blotting using an anti-R-GlcNAc monoclonal antibody (Abcam). _In vivo_ glycosylation assays were performed using _C.
rodentium_ and _S. enterica_ strains electroporated with plasmids expressing His-GshB or His-GshB(R256A). Transformed bacteria were grown overnight in the presence of 0.5 mM IPTG, harvested
using centrifugation, and then His-tagged proteins were purified as described above, and then subjected to western blotting using an anti-R-GlcNAc monoclonal antibody (Abcam). BACTERIAL
GROWTH ASSAYS Overnight cultures were used to inoculate 50 ml of LB medium. H2O2 (2.4 mM for _C. rodentium_ or 12.0 mM for _S. enterica_) was added to cultures when they reached an OD600 of
0.3 and bacterial growth was monitored for 10–16 h at 37 °C. GSH QUANTIFICATION FROM BACTERIAL LYSATES Bacteria were harvested by centrifugation, lysed in 50 mM Tris-HCl pH 7.4 supplemented
with 0.5 ug/ml lysozyme, and recentrifuged. The supernatant was incubated with 1 mM CDNB and 1 µM GST. Absorbance was recorded at 340 nm every 20 seconds for 10 minutes and OD340 data were
converted into molar concentrations of GSH using a GST standard curve24. _IN-VITRO_ GSH ASSAYS His-GshB or His-GshB(R256A) were purified over Ni-NTA slurries after they were respectively
co-expressed with either NleB-FLAG or NleB(AAA)-FLAG in _E. coli_ BL21(DE3) cells. An _in vitro_ GSH assay was then prepared by first incubating GshA (1 µM) with 5 mM glycine, 5 mM cysteine,
and 5 mM glutamate in a reaction buffer containing 50 mM Tris HCl pH 7.4, 1 mM DTT, 1 mM MgCl2, 1 mM ATP, and 3% DMSO. After 1 h incubation at 37 °C, the purified GshB proteins (50 nM),
along with 100 nM GST and 1 mM CDNB were added, and glutathione formation was monitored by reading the absorbance at 340 nm as a function of time. STATISTICAL ANALYSES Bacterial growth
assays were analyzed using non-linear regression followed by Dunn’s multiple comparison testing. GSH assays were analyzed using linear regression. p-values <0.05 were considered
significant. REFERENCES * Gao, X. _et al_. NleB, a bacterial effector with glycosyltransferase activity, targets GAPDH function to inhibit NF-kappaB activation. _Cell host microbe_ 13,
87–99, https://doi.org/10.1016/j.chom.2012.11.010 (2013). Article CAS PubMed PubMed Central Google Scholar * Li, S. _et al_. Pathogen blocks host death receptor signalling by arginine
GlcNAcylation of death domains. _Nat._ 501, 242–246, https://doi.org/10.1038/nature12436 (2013). Article ADS CAS Google Scholar * Pearson, J. S. _et al_. A type III effector antagonizes
death receptor signalling during bacterial gut infection. _Nat._ 501, 247–251, https://doi.org/10.1038/nature12524 (2013). Article ADS CAS Google Scholar * Ding, J. _et al_. Structural
and Functional Insights into Host Death Domains Inactivation by the Bacterial Arginine GlcNAcyltransferase Effector. _Molecular cell_ 74, 922-935.e926,
https://doi.org/10.1016/j.molcel.2019.03.028 (2019). Article Google Scholar * Esposito, D. _et al_. Structural basis for the glycosyltransferase activity of the Salmonella effector SseK3.
_J. Biol. Chem._ 293, 5064–5078, https://doi.org/10.1074/jbc.RA118.001796 (2018). Article CAS PubMed PubMed Central Google Scholar * Park, J. B. _et al_. Structural basis for arginine
glycosylation of host substrates by bacterial effector proteins. _Nat. Commun._ 9, 4283, https://doi.org/10.1038/s41467-018-06680-6 (2018). Article ADS CAS PubMed PubMed Central Google
Scholar * Deng, W. _et al_. Assembly, structure, function and regulation of type III secretion systems. _Nat. Rev. Microbiol._ 15, 323–337, https://doi.org/10.1038/nrmicro.2017.20 (2017).
Article CAS PubMed Google Scholar * Wagner, S. _et al_. Bacterial type III secretion systems: a complex device for the delivery of bacterial effector proteins into eukaryotic host cells.
_FEMS microbiology letters_ 365, https://doi.org/10.1093/femsle/fny201 (2018). * Feldman, M. F. & Cornelis, G. R. The multitalented type III chaperones: all you can do with 15 kDa.
_FEMS microbiology Lett._ 219, 151–158, https://doi.org/10.1016/s0378-1097(03)00042-9 (2003). Article CAS Google Scholar * Young, C. & Nester, E. W. Association of the virD2 protein
with the 5’ end of T strands in Agrobacterium tumefaciens. _J. Bacteriol._ 170, 3367–3374, https://doi.org/10.1128/jb.170.8.3367-3374.1988 (1988). Article CAS PubMed PubMed Central
Google Scholar * Pelczar, P., Kalck, V., Gomez, D. & Hohn, B. Agrobacterium proteins VirD2 and VirE2 mediate precise integration of synthetic T-DNA complexes in mammalian cells. _EMBO
Rep._ 5, 632–637, https://doi.org/10.1038/sj.embor.7400165 (2004). Article CAS PubMed PubMed Central Google Scholar * Kusmierek, M. _et al_. A bacterial secreted translocator hijacks
riboregulators to control type III secretion in response to host cell contact. _PLoS Pathog._ 15, e1007813, https://doi.org/10.1371/journal.ppat.1007813 (2019). Article CAS PubMed PubMed
Central Google Scholar * Lassak, J. _et al_. Arginine-rhamnosylation as new strategy to activate translation elongation factor P. _Nat. Chem. Biol._ 11, 266–270,
https://doi.org/10.1038/nchembio.1751 (2015). Article CAS PubMed PubMed Central Google Scholar * Lassak, J., Koller, F., Krafczyk, R. & Volkwein, W. Exceptionally versatile -
arginine in bacterial post-translational protein modifications. _Biol. Chem._ 400, 1397–1427, https://doi.org/10.1515/hsz-2019-0182 (2019). Article CAS PubMed Google Scholar * Pan, M.
_et al_. Synthesis of and specific antibody generation for glycopeptides with arginine N-GlcNAcylation. _Angew. Chem. Int. Ed. Engl._ 53, 14517–14521, https://doi.org/10.1002/anie.201407824
(2014). Article CAS PubMed Google Scholar * Scott, N. E. _et al_. The bacterial arginine glycosyltransferase effector NleB preferentially modifies Fas-associated death domain protein
(FADD). _J. Biol. Chem._ 292, 17337–17350, https://doi.org/10.1074/jbc.M117.805036 (2017). Article CAS PubMed PubMed Central Google Scholar * El Qaidi, S. _et al_. NleB/SseK effectors
from Citrobacter rodentium, Escherichia coli, and Salmonella enterica display distinct differences in host substrate specificity. _J. Biol. Chem._ 292, 11423–11430,
https://doi.org/10.1074/jbc.M117.790675 (2017). Article CAS PubMed PubMed Central Google Scholar * Lushchak, V. I. Glutathione homeostasis and functions: potential targets for medical
interventions. _J. amino acids_ 2012, 736837, https://doi.org/10.1155/2012/736837 (2012). Article CAS PubMed PubMed Central Google Scholar * Song, M. _et al_. Low-molecular-weight
thiol-dependent antioxidant and antinitrosative defences in Salmonella pathogenesis. _Mol. microbiology_ 87, 609–622, https://doi.org/10.1111/mmi.12119 (2013). Article CAS Google Scholar
* Wongsaroj, L. _et al_. Pseudomonas aeruginosa glutathione biosynthesis genes play multiple roles in stress protection, bacterial virulence and biofilm formation. _PLoS one_ 13, e0205815,
https://doi.org/10.1371/journal.pone.0205815 (2018). Article CAS PubMed PubMed Central Google Scholar * Potter, A. J., Trappetti, C. & Paton, J. C. Streptococcus pneumoniae uses
glutathione to defend against oxidative stress and metal ion toxicity. _J. Bacteriol._ 194, 6248–6254, https://doi.org/10.1128/jb.01393-12 (2012). Article CAS PubMed PubMed Central
Google Scholar * Zhang, Y. _et al_. Glutathione Activates Type III Secretion System Through Vfr in Pseudomonas aeruginosa. _Frontiers in Cellular and Infection Microbiology_ 9,
https://doi.org/10.3389/fcimb.2019.00164 (2019). * Reniere, M. L. _et al_. Glutathione activates virulence gene expression of an intracellular pathogen. _Nat._ 517, 170–173,
https://doi.org/10.1038/nature14029 (2015). Article ADS CAS Google Scholar * Habig, W. H., Pabst, M. J. & Jakoby, W. B. Glutathione S-transferases. The first enzymatic step in
mercapturic acid formation. _J. Biol. Chem._ 249, 7130–7139 (1974). CAS PubMed Google Scholar * Hebrard, M., Viala, J. P., Meresse, S., Barras, F. & Aussel, L. Redundant hydrogen
peroxide scavengers contribute to Salmonella virulence and oxidative stress resistance. _J. Bacteriol._ 191, 4605–4614, https://doi.org/10.1128/JB.00144-09 (2009). Article CAS PubMed
PubMed Central Google Scholar * Horst, S. A. _et al_. Thiol peroxidase protects Salmonella enterica from hydrogen peroxide stress _in vitro_ and facilitates intracellular growth. _J.
Bacteriol._ 192, 2929–2932, https://doi.org/10.1128/JB.01652-09 (2010). Article CAS PubMed PubMed Central Google Scholar * Karash, S., Liyanage, R., Qassab, A., Lay, J. O. Jr. &
Kwon, Y. M. A Comprehensive Assessment of the Genetic Determinants in Salmonella Typhimurium for Resistance to Hydrogen Peroxide Using Proteogenomics. _Sci. Rep._ 7, 17073,
https://doi.org/10.1038/s41598-017-17149-9 (2017). Article ADS CAS PubMed PubMed Central Google Scholar * Thomas, N. A., Deng, W., Baker, N., Puente, J. & Finlay, B. B.
Hierarchical delivery of an essential host colonization factor in enteropathogenic Escherichia coli. _J. Biol. Chem._ 282, 29634–29645, https://doi.org/10.1074/jbc.M706019200 (2007). Article
CAS PubMed Google Scholar * Ramu, T. _et al_. A novel C-terminal region within the multicargo type III secretion chaperone CesT contributes to effector secretion. _J. Bacteriol._ 195,
740–756, https://doi.org/10.1128/JB.01967-12 (2013). Article CAS PubMed PubMed Central Google Scholar * Hara, T., Kato, H., Katsube, Y. & Oda, J. A pseudo-michaelis quaternary
complex in the reverse reaction of a ligase: structure of Escherichia coli B glutathione synthetase complexed with ADP, glutathione, and sulfate at 2.0A resolution. _Biochem._ 35,
11967–11974, https://doi.org/10.1021/bi9605245 (1996). Article CAS Google Scholar * Lopez-Campistrous, A. _et al_. Localization, annotation, and comparison of the Escherichia coli K-12
proteome under two states of growth. _Mol. Cell Proteom._ 4, 1205–1209, https://doi.org/10.1074/mcp.D500006-MCP200 (2005). Article CAS Google Scholar * Ude, S. _et al_. Translation
elongation factor EF-P alleviates ribosome stalling at polyproline stretches. _Sci._ 339, 82–85, https://doi.org/10.1126/science.1228985 (2013). Article ADS CAS Google Scholar * Urbanus,
M. L. _et al_. Diverse mechanisms of metaeffector activity in an intracellular bacterial pathogen, Legionella pneumophila. _Mol. Syst. Biol._ 12, 893, https://doi.org/10.15252/msb.20167381
(2016). Article CAS PubMed PubMed Central Google Scholar * Qaidi, S. E. & Hardwidge, P. R. ABC cloning: An efficient, simple, and rapid restriction/ligase-free method. _MethodsX_ 6,
316–321, https://doi.org/10.1016/j.mex.2019.02.007 (2019). Article PubMed PubMed Central Google Scholar * Datsenko, K. A. & Wanner, B. L. One-step inactivation of chromosomal genes
in Escherichia coli K-12 using PCR products. _Proc. Natl Acad. Sci. U S Am._ 97, 6640–6645, https://doi.org/10.1073/pnas.120163297 (2000). Article ADS CAS Google Scholar * Karmali, M. A.
_et al_. Association of genomic O island 122 of Escherichia coli EDL 933 with verocytotoxin-producing Escherichia coli seropathotypes that are linked to epidemic and/or serious disease. _J.
Clin. Microbiol._ 41, 4930–4940, https://doi.org/10.1128/jcm.41.11.4930-4940.2003 (2003). Article CAS PubMed PubMed Central Google Scholar * Scott, N. E. _et al_. Simultaneous
glycan-peptide characterization using hydrophilic interaction chromatography and parallel fragmentation by CID, higher energy collisional dissociation, and electron transfer dissociation MS
applied to the N-linked glycoproteome of Campylobacter jejuni. _Mol. Cell Proteom._ 10, M000031–MCP000201, https://doi.org/10.1074/mcp.M000031-MCP201 (2011). Article CAS Google Scholar *
Rappsilber, J., Mann, M. & Ishihama, Y. Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using StageTips. _Nat. Protoc._ 2,
1896–1906, https://doi.org/10.1038/nprot.2007.261 (2007). Article CAS PubMed Google Scholar * Hughes, C. S. _et al_. Single-pot, solid-phase-enhanced sample preparation for proteomics
experiments. _Nat. Protoc._ 14, 68–85, https://doi.org/10.1038/s41596-018-0082-x (2019). Article CAS PubMed Google Scholar * Cox, J. & Mann, M. MaxQuant enables high peptide
identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. _Nat. Biotechnol._ 26, 1367–1372, https://doi.org/10.1038/nbt.1511 (2008). Article
CAS PubMed Google Scholar * Cox, J. _et al_. Accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction, termed MaxLFQ. _Mol. Cell
Proteom._ 13, 2513–2526, https://doi.org/10.1074/mcp.M113.031591 (2014). Article CAS Google Scholar * Tyanova, S. _et al_. The Perseus computational platform for comprehensive analysis of
(prote)omics data. _Nat. Methods_ 13, 731–740, https://doi.org/10.1038/nmeth.3901 (2016). Article CAS PubMed Google Scholar * Brademan, D. R., Riley, N. M., Kwiecien, N. W. & Coon,
J. J. Interactive Peptide Spectral Annotator: A Versatile Web-based Tool for Proteomic Applications. _Mol. Cell Proteom._ 18, S193–S201, https://doi.org/10.1074/mcp.TIR118.001209 (2019).
Article CAS Google Scholar * Luperchio, S. A. _et al_. Citrobacter rodentium, the causative agent of transmissible murine colonic hyperplasia, exhibits clonality: synonymy of C. rodentium
and mouse-pathogenic Escherichia coli. _J. Clin. Microbiol._ 38, 4343–4350 (2000). Article CAS Google Scholar * Deng, W. _et al_. Dissecting virulence: systematic and functional analyses
of a pathogenicity island. _Proc. Natl Acad. Sci. U S Am._ 101, 3597–3602, https://doi.org/10.1073/pnas.0400326101 (2004). Article ADS CAS Google Scholar * El Qaidi, S. _et al_.
High-Throughput Screening for Bacterial Glycosyltransferase Inhibitors. _Front. Cell Infect. Microbiol._ 8, 435, https://doi.org/10.3389/fcimb.2018.00435 (2018). Article CAS PubMed PubMed
Central Google Scholar Download references ACKNOWLEDGEMENTS The project described was supported by grant number AI127973 (PRH) from the National Institute of Allergy and Infectious
Diseases (NIAID). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIAID. This work was also supported by National Health
and Medical Research Council of Australia (NHMRC) project grants awarded to NES (APP1100164). We thank the Melbourne Mass Spectrometry and Proteomics Facility of The Bio21 Molecular Science
and Biotechnology Institute at The University of Melbourne for the support of mass spectrometry analysis. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * College of Veterinary Medicine, Kansas
State University, Manhattan, KS, 66506, USA Samir El Qaidi, Michael P. Hays, Shelby Watkins & Philip R. Hardwidge * Department of Microbiology and Immunology, University of Melbourne
within the Peter Doherty Institute for Infection and Immunity, Melbourne, 3000, Australia Nichollas E. Scott * Department of Biochemistry and Molecular Biophysics, Kansas State University,
Manhattan, KS, 66506, USA Brian V. Geisbrecht Authors * Samir El Qaidi View author publications You can also search for this author inPubMed Google Scholar * Nichollas E. Scott View author
publications You can also search for this author inPubMed Google Scholar * Michael P. Hays View author publications You can also search for this author inPubMed Google Scholar * Brian V.
Geisbrecht View author publications You can also search for this author inPubMed Google Scholar * Shelby Watkins View author publications You can also search for this author inPubMed Google
Scholar * Philip R. Hardwidge View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS S.E., N.S., M.P.H. and S.W. performed the experiments N.S.,
B.V.G. and P.R.H. analyzed the data, wrote the main manuscript text and prepared figures. All authors reviewed the data. CORRESPONDING AUTHOR Correspondence to Philip R. Hardwidge. ETHICS
DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in
published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPORTING INFORMATION. SUPPORTING INFORMATION2. SUPPORTING INFORMATION3. SUPPORTING INFORMATION4. SUPPORTING
INFORMATION5. SUPPORTING INFORMATION6. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing,
adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons
license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a
credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted
use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT
THIS ARTICLE CITE THIS ARTICLE El Qaidi, S., Scott, N.E., Hays, M.P. _et al._ An intra-bacterial activity for a T3SS effector. _Sci Rep_ 10, 1073 (2020).
https://doi.org/10.1038/s41598-020-58062-y Download citation * Received: 19 November 2019 * Accepted: 10 January 2020 * Published: 23 January 2020 * DOI:
https://doi.org/10.1038/s41598-020-58062-y SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative