Estimation of dietary 14c dose coefficient using 13c-labelled compound administration analysis
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Carbon-14 released from nuclear facilities has been assessed to contribute significantly to the radiation dose that people are exposed to through the food chain. However, the
current dose coefficient for members of public, which is the ratio of the 50-year committed effective dose to ingested 1 Bq 14C, recommended by the International Commission on Radiological
Protection (ICRP) is not based on experimental human metabolic data for 14C in nutrients and diet. Therefore, to validate the coefficient, we administered 13C-labelled nutrients consisting
of four amino acids, three fatty acids, and one monosaccharide to volunteers as substitutes for 14C labelled nutrients and measured the 13C concentration in various excreta samples. Although
metabolic models were constructed from the excretion data, a significant fraction of administered 13C was not recovered from some nutrients. The dose coefficients of 14C in uniformly
labelled Japanese diet, which were estimated under several assumptions about the unrecoverable fraction, varied from (6.2 ± 0.9) × 10–11 to (8.9 ± 4.4) × 10–10 Sv Bq−1 and were approximately
comparable to the current value of 5.8 × 10–10 Sv Bq−1 recommended by the ICRP. Further studies are necessary to elucidate the metabolism of 14C in various nutrients in the unrecoverable
fraction. SIMILAR CONTENT BEING VIEWED BY OTHERS ESTIMATION OF RADIATION DOSE FROM INGESTED TRITIUM IN HUMANS BY ADMINISTRATION OF DEUTERIUM-LABELLED COMPOUNDS AND FOOD Article Open access
02 February 2021 EFFECTIVE DOSES RECEIVED BY THE GASTROINTESTINAL TRACT COMPARTMENTS OF ADULTS DUE TO FOOD INTAKE IN EGYPT Article Open access 28 January 2025 DIETARY EXPOSURE OF
RADIONUCLIDES AND HEAVY METALS IN ADULT RESIDENTS IN A HIGH BACKGROUND NATURAL RADIATION AREA USING DUPLICATE DIET METHOD Article Open access 06 October 2022 INTRODUCTION Carbon-14 is
released from places such as nuclear fuel reprocessing facilities and nuclear power plants1. It is a major radionuclide contributing to the radiation dose to people around the nuclear fuel
reprocessing facilities1,2,3. The dose coefficient of 14C, the ratio of 50-year committed effective dose to ingested 1 Bq 14C, is an important parameter for the dose evaluation. The
International Commission on Radiological Protection (ICRP), which provides recommendations and guidance on radiological protection, has developed models describing the biokinetics of
radionuclides for the calculation of doses from data of intakes or bioassay samples. For estimating the dose coefficient of 14C in diet of members of public, the ICRP has utilized a generic
one-compartment model for organic carbon metabolism4, whereas a multi-compartment model was used in a recent publication for unspecified 14C compounds ingested by workers5. The
one-compartment model for members of public assumes that 14C is uniformly distributed throughout all body organs and tissues following entry into the systemic circulation. The organic-carbon
compartment of the model has a half-life of 40 days, which is derived from the mass balance of carbon in the reference man of 70 kg body weight with body carbon weight of 16 kg and carbon
intake and excretion of 300 g carbon per day each6. The dose coefficient of organic 14C in the diet (5.8 × 10−10 Sv Bq−1) was defined using that metabolic model7, which is not based on
actual experimental data obtained from 14C in foods or component compounds of major nutrients. Therefore, experimental data of 14C in those compounds are needed to validate the generic
one-compartment model of the ICRP or revise it in future publications for members of public following publications for workers8. In rat tissue and organs, some studies have examined the
biokinetics of 14C incorporated into foods or component molecule of major nutrient, e.g., single oral administration of [14C(U)]-glucose, [14C(U)]-glycine, [14C(U)]-leucine, [14C(U)]-lysine,
[1–14C]-oleic acid, [1-14C]palmitic acid, and [2-14C]-thymidine9; and continuous ingestion of 14C-wheat10, [14C(U)]-glycine, [14C(U)]-leucine, and 14C-rapeseed11. In sheep, Crout _et al_.
reported the biokinetics of 14C after oral administration of 14C-glucose12. However, most of the human data has been restricted to the 14C in drug molecules in biomedical studies13 and
diagnostic use such as 14C-glycocholic acid and 14C-xylose breath tests14, and only few investigations have reported the biokinetics of 14C in component molecule of major nutrients.
Malmendier _et al_.15 examined the metabolism of 14C in intravenously administered [1-14C]-glucose, [1-14C]-glycerol, and [1-14C]-palmitate for 24 h in breath in normal and hyperlipemic
patients. Simmons _et al_.16 reported the 14C excretion in urine and breath until 3 and 7 days, respectively, after intravenous [1-14C]-alanine administration for human volunteers. However,
both studies monitored the breath for a short time of up to 7 days after the administration. Berlin and Tolbert17 reported the breath excretion of 14C following intravenous injection of
glycine-[2-14C] decreased in four successive phases with half-lives of 0.12, 0.97, 6.1, and 71.5 days. Stenström _et al_.18 orally administered 14C-triolein and found two phase excretions in
the breath with half-lives of 2 and several hundred days. Gunnarsson _et al_.19 also reported that the excretion of 14C in breath after oral 14C-triolein administration decreased in three
phases with half-lives of 0.04, 2, and 150 days. Although the data are limited, several studies have reported fractions of excreta with longer half-lives than that of the ICRP generic
one-compartment model as described above. Ingested carbon is excreted via several physiological routes including in the breath, urine, faeces, hair, nails, epithelial cells of the skin and
mucous membrane, mucus, and other secretions. The ICRP has classified the routes into four excretion pathways, breath, urine, faeces, and others (other*, hereafter) and estimated the
distribution ratio of carbon via the pathways in their reference man6. However, the previous studies on carbon excretion in humans described above examined only the respiration and urinary
pathways. Regarding the other* pathway, Stenström _et al_. reported the specific activity of 14C in hair from personnel at a research laboratory and pointed out the possibility of using hair
as a bioassay sample20. To the best of our knowledge, no data has been reported for the human body on the metabolism of 14C incorporated into compounds covering a broad range of nutrients
and food, considering every four excretion routes of the ICRP. We previously reported the biokinetics of carbon in the human body estimated using data of the 13C/12C ratio in breath, urine,
faeces, and hair, representing carbon excretion through the breath, urine, faeces, and other* pathways, respectively, after a single oral administration of 13C-labelled glucose to three
healthy male volunteers21. The 13C/12C ratio in breath, urine, and hair samples was measured for up to ~112 days after administration. The excretion of 13C in the evaluated pathways
decreased in two exponential phases with fast and slow rates, and the 13C/12C ratio of the other* pathway was higher than that of the breath and urine pathways toward the end of the
experiment. Although this finding suggests that the carbon pool of the other* pathway had a longer half-life than that of the breath and urine pathways, we could not get the statistically
significant 13C half-life of the slow decreasing fraction in the other* pathway. In this paper, we report the results of experiments administering 13C-labelled four amino acids and three
fatty acids to volunteers. First, 13C-labelled leucine and palmitic acid were orally administered once to the volunteers and the excretion through the four routes of interest were measured
for up to ~160 day after the administration. Then, the contribution of carbon pools of each route to the cumulative burden of 14C for 50 years was calculated assuming the same metabolism of
13C and 14C. Since the urine and faeces routes did not have significant contributions, we focused on only the breath and other* routes in experiments on the remaining nutrient components. In
addition, once daily dosing administered four successive times, instead of the previously used single administration, was adopted for a more accurate measurement of 13C excretion in the
final stage of the experiments. An experiment on 13C-labled glucose administered four times was also conducted and, finally, the dose coefficient of 14C in the Japanese diet was estimated
using integrated biokinetic models of each nutrient component. RESULTS AND DISCUSSION DESIGN OF EXPERIMENTS We selected the following amino acids as representative of proteins: leucine from
branched chain amino acids, glutamic acid from major amino acids, glycine from collagen components, and phenylalanine from aromatic amino acids. Palmitic, oleic, and linoleic acids were
selected as representative saturated, mono-unsaturated, and poly-unsaturated fatty acids, respectively while carbohydrates were represented by glucose. All carbon atoms of the compounds used
were labelled with 13C (Cambridge Isotopes, Inc., Tewksbury). The compounds were administered to healthy volunteers and samples of various excreta were collected from them and analysed for
13C. All the 13C/12C ratio data presented in this report are shown as increments from the background values measured for the samples before administration, and normalized to the same dose
per body weight (1 g 13C/70 kg body weight) prior to the following analysis. When the 13C/12C ratio under the detection limits, <95% confidence limit of the background, was observed, the
data of the detection day and after it were not used for the subsequent numerical analysis. The detection limit mostly depended on the amount of 13C administered per body weight and
individual difference. SELECTION OF MAJOR EXCRETION ROUTES OF 13C IN AMINO ACID AND FATTY ACID We orally administered 13C-labelled leucine or palmitic acid to three male volunteers who were
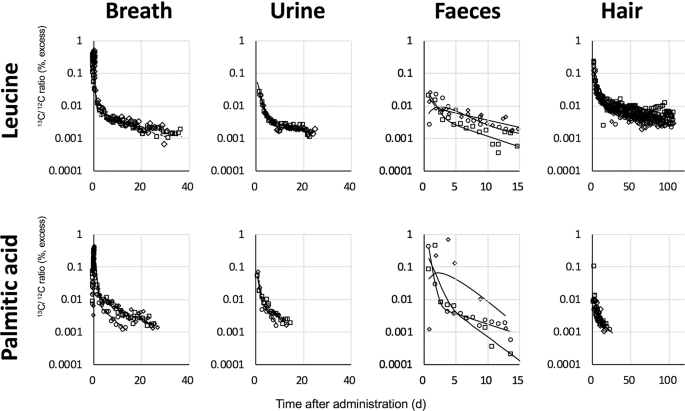
sedentary workers and then collected their breath, urine, faeces, and hair samples at different times depending on the compounds and excreta (Fig. 1). As shown in Figure 1, the 13C/12C ratio
of the breath and hair samples increased after administration then decreased in two exponential phases. The peak time of breath samples from the leucine-administered group (0.11 ± 0.02
days, mean ± standard deviation) was earlier than that of the palmitic acid-administered group (0.30 ± 0.03 days, P < 0.01), suggesting that the compounds had different absorption rates
from the alimentary tract (Fig. S1 in the supplementary information). The peak time of the 13C/12C ratio in faeces samples after administration of labelled leucine and palmitic acid varied
from 0.75 to 1.9 days and from 0.75 to 3.75 days among subjects, respectively. The excretion rate of administered 13C into the faeces is considered to depend on subject-specific values such
as residence time of faeces in the colon, which was independent of the administered compounds. For each compound, we developed a metabolic model comprising four carbon excretion pathways:
breath, urine, faeces, and other* (Fig. 2). Since the 13C/12C ratios of each excreta sample showed a dual exponential decrease after peaking, two compartments representing fast and slow
carbon metabolism were set for each of the four excretion pathways. However, every compartment in those models does not represent any tissue and organ. In addition, one upstream compartment
was used for each pathway to fit the prediction curve of the model to the increase phase observed in the breath and faeces samples immediately after administration (Fig. 1 and Fig. S1). The
rate of 13C excretion for each pathway (_E__m_, g d−1) is given by the following equations: $${E}_{m}=\sum
_{n}{k}_{mn}\frac{{d}_{m}{d}_{mn}{k}_{m}}{{k}_{m}-{k}_{mn}}({e}^{-{k}_{mn}t}-{e}^{-{k}_{m}t})$$ (1) where, _m_ is the excretion pathway symbol of _B, U, F_, and _O_ corresponding to the
excretion pathways of breath, urine, faeces, and other*, respectively; _n_ is the symbol of _f_ and _s_ corresponding to the compartments with fast and slow metabolism, respectively; _k__m_
and _k__mn_ are the excretion rate constants from compartment _C__m_ and _C__mn_, respectively; _d__m_ is the distribution ratio of 13C into compartment _C__m_ to the administered 13C;
_d__mn_ is the distribution ratio of 13C into compartment _C__mn_ to 13C from compartment _C__m_; _R__r_ is the recovery ratio of administered 13C. The sum of distribution ratios of
compartments with fast and slow metabolism was fixed to unity (i.e. _d__Bf_ + _d__Bs_ = 1). Since the sum of the distribution ratios of each pathway (_d__B_ + _d__U_ + _d__F_ + _d__O_) was
not forced to unity, to prevent underestimation of these parameters, _R__r_ was over unity for some cases. The parameter values were estimated using the data of each volunteer using a least
square method (Table 1). Although some parameters were not statistically significant (p > 0.05, most parameters for the urine, faeces, and other* pathway in the palmitic acid model, and
in the faeces pathway in the leucine model), we included those parameters in the following analysis. We calculated the 50-year cumulative body burdens of 13C from orally ingested 1 g 13C in
leucine and palmitic acid using the above models. Then, the variation of the burden in response to a ± 10% change of each parameter was calculated using the mixed data from all subjects for
each compound group. The results are shown in Fig. 3 for the top 10 parameters causing larger variation and most were in the breath and other* pathways, whereas only two parameters causing
smaller variations in the faeces pathway were included. All the statistically insignificant parameters in the regression analysis were not sensitive to the burden. The contribution of urine
and faeces pathways to the burden from orally ingested 1 g of 13C in leucine were (0.37 ± 0.25)% and (0.7 ± 1.5)%, respectively, and those for palmitic acid were (0.038 ± 0.030)% and (0.034
± 0.070)%, respectively. Therefore, we concluded that the urine and faeces pathway could be omitted from the following experiment on carbon dynamics in our study. For glucose, we previously
reported the negligible contribution of the urine and faeces pathways to the cumulative burden21. METABOLISMS OF 13C ADMINISTERED AS VARIOUS 13C -LABELLED COMPOUNDS We also administered the
remaining target nutrient compounds,13C-labelled glutamic acid, glycine, phenylalanine, oleic acid, linoleic acid, and glucose to the volunteers who were healthy university students, three
males and females each, and then we collected only breath and hair samples. All volunteers were administered each compound once daily for 4 successive days. Figure 4 shows the 13C/12C ratio
of the breath and hair samples after the first administration of each 13C-labelled compound. The 13C/12C ratios of hair samples decreased faster in the groups of volunteers administered
oleic acid, linoleic acid, and glucose than in the other groups and reached the detection limit before the end of experiments. Consequently, we did not analyse parameters relating the slow
carbon compartment in the other* pathway for some subjects. In contrast, the 13C/12C ratios of the groups of volunteers administered glycine and phenylalanine were kept relatively high
throughout the experimental period, and were higher than those in the breath samples of the same volunteers toward the end of the experiment. These results reflected the higher utilization
of carbon in amino acids for protein synthesis such as in hair growth than carbon in fatty acids and monosaccharides. The results of leucine and palmitic acid-administered group also showed
a similar tendency (Fig. 1). However, amino acids in samples from volunteers in the glutamic acid-administered group decreased faster than other amino acids did. This observation is
consistent with the reported high use of dietary glutamic acid in the small intestine22. The metabolic model was also developed for each compound. The values of parameters of the breath and
other* pathways were estimated and are summarized in Table 2. The parameters for subjects with only fast C compartment in the other* pathway were included in the summarization. Since the
difference of results between sexes was not clear, the average values for individual data of both sexes were also shown in Table 2 and discussed below. The estimated distribution ratio of
13C for the breath pathway (_d__B_) for leucine, glutamic acid, glycine, and phenylalanine were (68 ± 5)%, (84 ± 8)%, (80 ± 9)%, and (64 ± 7)%, respectively. Berlin and Tolbert17 reported an
86% recovery through the breath pathway after intravenous administration of glycine-[2-14C], which was consistent with the value of glycine in our experiment. Compared to glutamic acid and
glycine, smaller proportions of leucine and phenylalanine were excreted through the breath pathway while a considerable portion was excreted through the other* pathway (_d__O_, (30 ± 12)%
and (35 ± 8)%, respectively). Leucine stimulates protein synthesis via the mammalian target of rapamycin complex 1 (mTORC1) mediating signalling pathway23 and, therefore, it might activate
this pathway and consequently reduced its oxidation. However, such an effect has not been reported for phenylalanine. The distribution ratio of 13C of glucose in the breath pathway (_d__B_,
90 ± 15%) was similar to the value reported in our previous paper (86 ± 10%)21. The mean slow excretion rate constants (_k__Bs_) of the breath pathway varied from 0.061 to 0.21 day−1 among
the examined amino acids. These values were 5–20 times larger than the slow transfer coefficient of 0.0099 day−1 from the systemic tissue compartment to the carbon dioxide compartment in the
generic model for workers recommended by the ICRP-1345. However, the slow excretion constants (_k__Os_) of the other* pathway for the examined amino acids were from 0.0059 to 0.012 day−1
and comparable to the value of the slow compartment in the generic model for workers. Our result provides evidence to supports the existence of a carbon pool with a slow metabolic rate.
ESTIMATION OF DOSE COEFFICIENT FOR MEMBERS OF PUBLIC FROM INGESTED DIETARY 14C As shown in Table 2B,C, the recovery ratios (_R__r_) of the administered 13C in oleic and linoleic acids were
markedly small at 0.62 and 0.68, respectively, while most of the administered 13C in the other examined compounds including palmitic acid were recovered in our experiment (Tables 1 and 2).
Takeda _et al_.9 reported that the 14C concentration in adipose tissue in rats 100 days after administration of 14C-labelled oleic acid was three times higher than that of palmitic acid.
Although palmitic acid is a main constituent of adipose tissue fatty acids in humans24, in this study, we assumed that the unrecoverable fraction of 13C in both unsaturated fatty acids and
all nutrient compounds examined was stored in adipose tissue. We further presumed that the carbon in adipose tissue had a mean residence time of 1.6 years, as estimated by Arner _et al_.25
for adipocyte lipids by analysing 14C originating from nuclear weapon tests. Since the ICRP does not provide the tissue weighting factor for adipose tissue, we also set two assumptions for
this, 0 and 0.12 used for the remaining organs in the ICRP-103 guidelines26. The dose coefficients obtained with each assumption are called non conservative estimation (ER) and conservative
estimation (EC) hereafter. The recovered fraction was assumed to be distributed uniformly in the whole-body carbon with a tissue weighting factor of unity. In considering the possible
existence of additional carbon compartment, which was not detected in our study but have a longer half-life than that in the present slow carbon compartments, we adopted the EC as a moderate
estimation. In addition to these estimations, we calculated the most conservative estimation (EX) adopting the tissue weighting factor of unity for unrecovered carbon. We solely adopted the
value for the maximal estimation. Figure 5A shows the 50-year committed effective dose from 1 Bq of the 14C in labelled compounds after single ingestion calculated using each model
developed. The ER values of the amino acids were generally higher than those of the fatty acids, whereas the EC and EX values were the opposite. Glucose showed similar ER values to those of
the unsaturated fatty acids, whereas the EC and EX values are similar to those of amino acids. The assumption about the unrecoverable fraction played an extremely important role in our
estimation of the dose because of the assumed long half-life of unrecovered carbon. The relative contribution of each pathway including the unrecoverable fraction to the EC is shown in Fig.
5B. It is noteworthy that parameters of the urine and faeces pathway in the first experiment were used for the other compounds (details are shown below), and the contribution of these
pathways was very minor (<0.8%). As shown in Fig. 5B, the unrecoverable fraction contributed significantly to the committed dose when we adopted a tissue weighting factor of 0.12 to
adipose tissue. We estimated the dose coefficient for members of public based on ingestion of a Japanese diet27, which was uniformly labelled by 14C and had the nutritional composition shown
in Table 3. The nutrition in the diet was classified into groups which was represented by each single compound examined in our study. The proportion of carbon ingested as the nutrition
groups to total carbon ingestion rate was obtained by using literature data28,29 (details are shown below). The total daily ingestion of 300 g carbon per day of ICRP reference man was
divided by the proportion of carbon ingestion rate each and inputted to each single compound model. The dose coefficient was obtained by summing up the 50-year committed effective dose from
each representative compound comprising the diet with a total ingestion of 1 Bq 14C. The ER of the Japanese diet was estimated to be (6.2 ± 0.9) × 10−11 Sv Bq−1 (Fig. 5C), which was nine and
two times smaller than the current dose coefficient of 5.8 × 10−10 Sv Bq−1 for members of public as recommended by the ICRP-727 and 1.6 ×10−10 Sv Bq−1 for workers adopting the generic
multi-compartment model by ICRP-1345, respectively. The EC and EX were (5.6 ± 2.6) × 10−10 and (8.9 ± 4.4) × 10−10 Sv Bq−1, respectively (Fig. 5C). These conservative values were not
significantly different from the current dose coefficient for members of public7. As described before, there is no data about metabolism of carbon in a broad range of nutrients administered
orally, this work firstly provides the experimental base of dose coefficient for members of public. Since the unrecoverable fraction was very important for the estimation of the dose
coefficient in our research, further studies are necessary to clarify the metabolism of 14C in various nutrients. MATERIALS AND METHODS ADMINISTRATION OF 13C-LABELLED COMPOUNDS In the first
experiment to select the major excretion routes for carbon in amino and fatty acids, an aliquot of uniformly 13C-labelled leucine and palmitic acid (Cambridge Isotopes, Inc., Tewksbury) was
administered to healthy adult Japanese male sedentary workers aged 41 ± 9 and 40 ± 5 years old (n =3 each), respectively. The dose was 1.0 g 13C per person. We followed the administration
procedure described in a previous paper21. Briefly, the 13C-labelled compounds were orally ingested by each volunteer at 12:15 immediately before eating lunch on experimental day 0. An
additional 0.1 g of 13C in the compounds was similarly administered on day 112 after the initial administration to mark that day in the hair samples (see below). To avoid background
fluctuations in the 13C/12C ratio by over-eating of 13C-rich foods, nutritionally balanced foods were provided to the volunteers throughout the experimental period from day −7 to 112,
although they were able to eat other foods under certain circumstances. In the second experiment using various 13C-labelled compounds, uniformly 13C-labelled glutamic acid, glycine,
phenylalanine, oleic acid, linoleic acid, and glucose (Cambridge Isotopes, Inc., Tewksbury) were administered to healthy adult Japanese male and female volunteers (n = 3, each) aged 25 ± 7
years once a day at 12:05 on 4 successive days. The doses of those compounds were 8.9, 7.1, 11.9, 11.0, 16.4, and 35.6 mg 13C kg−1 day−1, respectively. Additional doses of 13C-labelled
compounds were administered on day 160 for marking the 13C/12C ratio of hair samples and the volunteers freely ate meals without calorific restrictions. SAMPLE COLLECTION AND DETERMINATION
OF 13C/12C RATIO In the first experiment, breath, urine, faeces, and hair samples were periodically collected and the 13C/12C ratio was analysed following the procedure described in a
previous paper21. Briefly, breath and urine samples were collected daily when the volunteers rose in the morning from day −7 to 112. Faecal samples were collected once before day 0 and at
every defecation from day 0 to 14. On day 0, additional breath and urine samples were collected frequently after administration. Five hair samples from each subject were collected on day 119
and used as representative samples of the other* pathway. Then, 10 cm of each hair starting from the root was cut into 1-mm sections and the 13C/12C ratio determination of each showed two
peaks in the 13C/12C ratio corresponding to the first and second administration of 13C-compounds. According to the section number of the two peaks, the experimental date for each section
between the two peaks was determined under an assumption of uniform hair growth. In the second experiment, breath samples were collected until day 160 and hair samples were obtained by
brushing on day 167. The 13C/12C ratio was measured using a mass spectrometer (Delta V Advantage; Thermo Electron, Waltham, MA, USA) coupled with an elemental analyser (FlashEA 1112NC;
Thermo Electron) and a gas chromatograph (MAT GC; Thermo Electron) for combusting organic samples and purifying CO2. Breath samples were collected by the volunteers themselves, and some
samples showed clearly inconceivable results such as similar 13C/12C ratios over considerable periods of breath sample collection, which may have been caused by collecting the samples on a
single day instead of once daily sampling on sequential days. The whole data of such individuals were discarded, which decreased the number of samples from three in Table 2. Some volunteers
also dropped out of the sampling procedure for various reasons. CONSTRUCTING MULTI-COMPARTMENT METABOLIC MODEL BY REGRESSION ANALYSIS The 13C distribution ratios and excretion rate constants
of all compartments in the four excretion pathways for leucine and palmitic acid were determined using the least squares fitting method with the statistical analysis software R version
2.15.2., while only parameters for the breath and other* pathway were determined for the other six examined compounds. The detailed procedures used were described in a previous published
paper21. When urine and hair samples had no increase phase, the values of _k__U_ and _k__O_ were fixed to those of _k__B_ obtained from the same volunteers in the regression analysis.
ESTIMATION OF DOSE COEFFICIENT FOR MEMBERS OF PUBLIC FROM DIETARY 14C The 50-year cumulative body burden of 14C after a single ingestion of 1 Bq of 14C in each compound (_B__C_ Bq d) was
calculated using the metabolic model and its parameters described above, under the assumption of the same metabolic behaviour of 13C and 14C. The parameters of the urine and faeces pathway
for leucine and palmitic acid were used for the other amino acids and fatty acids. For glucose, the parameters of the urine and faeces pathways reported in our previous paper21 were adopted.
The total cumulative burden was derived as a sum of burdens from the recoverable and unrecoverable fractions with designated proportions _R__r_ and 1−_R__r_, respectively. For the
recoverable fraction, the burden was calculated using following equation: $${B}_{C}\approx \sum _{m}\frac{{d}_{m}}{{k}_{m}}+\sum _{m}\sum _{n}\frac{{d}_{m}{d}_{mn}}{{k}_{mn}}$$ (2) where,
every _d__m_ was divided by _R__r_ when _R__r_ was larger than unity. The 50-year committed effective dose (Sv) from ingestion of 1 Bq 14C was calculated from the 50-year cumulative burden
by using the ratio of absorbed dose to 14C radioactivity, radiation weighting factor of beta-ray from 14C (1.0) and tissue weighting factors. As previously described, for the unrecoverable
fraction, we used a mean residence time of 1.6 years (excretion rate constant of 0.0017 d−1)25 and tissue weighting factors of 0, 0.12, and unity for the estimation of ER, EC, and EX,
respectively. The Japanese typical nutrition intake rates were cited from the annual nationwide National Health and Nutrition Survey conducted by the Ministry of Health, Labour and Welfare,
Japan27. In addition, we used the proportion of each amino acid as reported by Iwaya28 to obtain the ingestion rate of each amino acid group including branched chain amino acid (BCAA), major
amino acid, glycine, and aromatic amino acid (AAA) groups (Table 3). We assumed that the metabolism of each amino acid group was the same as that of the represented compound examined in our
study as shown in Table 3. The lipids and carbohydrates were also represented by each single compound. Then, the ingestion rate of carbon in each nutrition group was calculated according to
the weight percentage of carbon in the representative compound (Table 3)29, followed by obtaining the proportion of carbon ingestion rate of each group to the total carbon ingestion rate.
Then, the input to each compound model was calculated from the total carbon ingestion rate of 300 g carbon per day of ICRP reference man6 and the proportion of carbon ingestion rate.
Finally, the dose coefficient for members of public from dietary 14C was calculated by summing up the committed effective doses of 14C and weighting proportion of 14C in each compound up to
1 Bq 14C in the diet. ETHICAL CONSIDERATIONS The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in the prior approval of the Institute
for Environmental Sciences Review Board for Human Subject Experiments. Written informed consent was obtained from each volunteer prior to participation. DATA AVAILABILITY The datasets
generated and analysed during the current study are available from the corresponding author on a reasonable request. REFERENCES * Koarashi, J., Akiyama, K., Asano, T. & Kobayashi, H.
Chemical composition of 14C in airbone release from the Tokai reprocessing plant, Japan. _Radiat. Prot. Dosimetry._ 114, 551–555 (2005). Article CAS Google Scholar * MaCartney, M.,
Baxtre, M. S. & Scott, E. M. Carbon-14 discharged from the nuclear fuel cycle: 2 Local effects. _J. Environ. Radioact._ 8, 157–171 (1988). Article Google Scholar * Veluri, V. R.,
Boone, F. W. & Palms, J. M. The environmental impact of 14C released by a nuclear funnel-reprocessing plant. _Nucl. Safety._ 17, 580–590 (1976). CAS Google Scholar * International
Commission on Radiological Protection (ICRP), Limits for intakes of radionuclides by workers. ICRP Publication 30. Ann. ICRP 2 (Elsevier (1979). * International Commission on Radiological
Protection (ICRP), Occupational intakes of radionuclides: Part 1. ICRP Publication 134. Ann. ICRP 45 (Elsevier (2016). * International Commission on Radiological Protection (ICRP), Report on
the task group on reference man. ICRP Publication 23. Ann. ICRP (Elsevier (1975). * International Commission on Radiological Protection (ICRP), Age-dependent doses to the members of the
public from intake of radionuclides: Part 5 Compilation of ingestion and inhalation coefficients. ICRP Publication 72. Ann. ICRP 26 (Elsevier 1995). * Paquet, F., Bailey, M. R. &
Leggett, R. Assessment and interpretation of internal doses: uncertainty and variability. _Ann. ICRP._ 45, 202–214 (2016). Article CAS Google Scholar * Takeda, H., Fuma, S., Miyamoto, K.,
Nishimura, Y. & Inaba, J. Biokinetics and dose estimation of radiocarbon in rats. _Radiat. Prot. Dosimetry._ 79, 169–174 (1998). Article CAS Google Scholar * Takeda, H. _et al_.
Biokinetics of radiocarbon ingested as a food in rats. _Health Phys._ 96, 587–593 (2009). Article CAS Google Scholar * Takeda, H. _et al_. Comparative biokinetics of radiocarbon ingested
as compounds or foods in rats. _Health Phys._ 99, 668–673 (2010). Article CAS Google Scholar * Crout, N. M., Mayes, R. W., Beresford, N. A., Lamb, C. S. & Howard, B. J. A metabolic
approach to simulating the dynamics of C-14, H-3 and S-35 in sheep tissues. _Radiat. Environ. Biophys._ 36, 243–250 (1998). Article CAS Google Scholar * Lappin, G. & Garner, R. C.
Current perspective of 14C-isotope measurement in biomedical accelerator mass spectrometry. _Anal. Bioanal. Chem._ 378, 356–364 (2004). Article CAS Google Scholar * Gunnarsson, M. _et
al_. Long term biokinetics and radiation exposure of patients undergoing 14C-glycocholic acid and 14C-xylose breath tests. _Cancer Biother. Radiopharm._ 22, 762–771 (2007). Article CAS
Google Scholar * Malmendier, C. L., Delcroix, C. & Berman, M. Interrelation in the oxidative metabolism of free fatty acids, glucose, and glycerol in normal and hyperlipemic patients.
_J. Clin. Invest._ 54, 461–476 (1974). Article CAS Google Scholar * Simmons, P. S., Nissen, S. & Haymond, M. W. Total body absorbed radiation dose from 1-14C-alanine for human
studies. _Health Phys._ 43, 561–565 (1982). Article CAS Google Scholar * Berlin, N. I. & Tolbert, B. M. Metabolism of glycine-2-14C in man: V. further consideration of pulmonary
excretion of 14CO2. _Proc. Soc. Exp. Biol. Med._ 88, 386–389 (1953). Article Google Scholar * Stenström, K. _et al_. Application of accelerator mass spectrometry (AMS) for high-sensitivity
measurements of 14CO2 in long term studies of fat metabolism. _Appl. Radiat. Isot._ 47, 417–422 (1996). Article Google Scholar * Gunnarsson, M. _et al_. Biokinetics and radiation
dosimetry for patients undergoing a glycerol tri[1-14C]oleate fat malabsorption breath test. _Appl. Radiat. Isot._ 58, 517–526 (2003). Article CAS Google Scholar * Stenström, K., Unkel,
I., Nilsson, C. M., Raaf, C. & Mattsson, S. The use of hair as an indicator of occupational 14C contamination. _Radiat. Environ. Biophys._ 49, 97–107 (2010). Article Google Scholar *
Masuda, T. _et al_. Biokinetics of 13C in the human body after oral administration of 13C-labeled glucose as an index for the biokinetics of 14C. _J. Radiol. Prot._ 36, 532–546 (2016).
Article CAS Google Scholar * Reeds, P. J., Burrin, D. G., Davis, T. A. & Stroll, B. Amino acid metabolism and the energetics of growth. _Arch. Tieremahr._ 51, 187–97 (1998). CAS
Google Scholar * Dickinson, J. M. _et al_. Mammalian target of rapamycin complex 1 activation is required for the stimulation of human skeletal muscle protein synthesis by essential amino
acids. _J. Nutr_ 141, 856–862 (2011). Article CAS Google Scholar * Insull, W., Lang, P. D., His, B. P. & Yoshimura, S. Studies of arteriosclerosis in Japanese and American men. _J.
Clin. Invest._ 48, 1313–1327 (1969). Article Google Scholar * Arner, P. _et al_. Dynamics of human adipose lipid turnover in health and metabolic disease. _Nature_ 478, 110–113 (2011).
Article ADS CAS Google Scholar * International Commission on Radiological Protection (ICRP). The 2007 recommendations of the International Commission on Radiological Protection. ICRP
Publication 103. Ann. ICRP 37 (Elsevier (1997). * Ministry of Health, Labour and Welfare, Japan. The National Health and Nutrition Survey, Japan [(accessed on 12th March 2020)]; Available
online: https://www.mhlw.go.jp/bunya/kenkou/kenkou_eiyou_chousa.html. (In Japanese). * Iwaya, M. Amino acid composition of dietary protein ingested by the Japanese and its nutritional
evaluation. _Jpn. J. Nutr. Diet._ 42, 297–304 (1984). Article Google Scholar * Nakamura, M. & Yuyama, Y. Development of a composition database for various types of biomass. _Tech.
Report NIRE_ 203, 57–80 (2005). Google Scholar Download references ACKNOWLEDGEMENTS We wish to express our sincere gratitude to the volunteers for their willing and extended cooperation.
This study was performed under a contract with the government of Aomori Prefecture, Japan. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Institute for Environmental Sciences, Aomori, Japan
Tsuyoshi Masuda, Kensaku Matsushita, Yasuhiro Tako, Yuichi Takaku & Shun’ichi Hisamatsu * Hirosaki Gakuin University, Aomori, Japan Toshitada Yoshioka * Kyoto University, Osaka, Japan
Tomoyuki Takahashi * National Institute of Radiological Sciences, Chiba, Japan Hiroshi Takeda * University of Tokyo, Tokyo, Japan Hideo Hatta Authors * Tsuyoshi Masuda View author
publications You can also search for this author inPubMed Google Scholar * Toshitada Yoshioka View author publications You can also search for this author inPubMed Google Scholar * Tomoyuki
Takahashi View author publications You can also search for this author inPubMed Google Scholar * Hiroshi Takeda View author publications You can also search for this author inPubMed Google
Scholar * Hideo Hatta View author publications You can also search for this author inPubMed Google Scholar * Kensaku Matsushita View author publications You can also search for this author
inPubMed Google Scholar * Yasuhiro Tako View author publications You can also search for this author inPubMed Google Scholar * Yuichi Takaku View author publications You can also search for
this author inPubMed Google Scholar * Shun’ichi Hisamatsu View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS T.M. conceived the idea and
designed the study; H.T. participated in the study design; T.M., K.M. and T.Y. acquired the data; T.M. and T.T. analysed the data; H.H., Ya. T. and Yu. T. participated in the study
coordination; T.M. and S.H. interpreted the data and wrote the text; H.T., T.T., H.H., Ya. T. and Yu. T., commented on the manuscript writing; All authors critically reviewed the data and
the manuscript; SH supervised the study. CORRESPONDING AUTHOR Correspondence to Tsuyoshi Masuda. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests.
ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION
SUPPLEMENTARY INFORMATION. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation,
distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and
indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to
the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will
need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE
CITE THIS ARTICLE Masuda, T., Yoshioka, T., Takahashi, T. _et al._ Estimation of dietary 14C dose coefficient using 13C-labelled compound administration analysis. _Sci Rep_ 10, 8156 (2020).
https://doi.org/10.1038/s41598-020-64954-w Download citation * Received: 16 January 2020 * Accepted: 24 April 2020 * Published: 18 May 2020 * DOI: https://doi.org/10.1038/s41598-020-64954-w
SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to
clipboard Provided by the Springer Nature SharedIt content-sharing initiative