Individual calculation of effective dose and risk of malignancy based on monte carlo simulations after whole body computed tomography
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Detailed knowledge about radiation exposure is crucial for radiology professionals. The conventional calculation of effective dose (ED) for computed tomography (CT) is based on dose
length product (DLP) and population-based conversion factors (k). This is often imprecise and unable to consider individual patient characteristics. We sought to provide more precise and
individual radiation exposure calculation using image based Monte Carlo simulations (MC) in a heterogeneous patient collective and to compare it to phantom based MC provided from the
National Cancer Institute (NCI) as academic reference. Dose distributions were simulated for 22 patients after whole-body CT during Positron Emission Tomography-CT. Based on MC we calculated
individual Lifetime Attributable Risk (LAR) and Excess Relative Risk (ERR) of cancer mortality. EDMC was compared to EDDLP and EDNCI. EDDLP (13.2 ± 4.5 mSv) was higher compared to EDNCI
(9.8 ± 2.1 mSv) and EDMC (11.6 ± 1.5 mSv). Relative individual differences were up to −48% for EDMC and −44% for EDNCI compared to EDDLP. Matching pair analysis illustrates that young age
and gender are affecting LAR and ERR significantly. Because of these uncertainties in radiation dose assessment automated individual dose and risk estimation would be desirable for dose
monitoring in the future. SIMILAR CONTENT BEING VIEWED BY OTHERS VALIDATION OF SSDE CALCULATION IN A MODERN CT SCANNER AND CORRELATION WITH EFFECTIVE DOSE Article Open access 19 February
2025 CHARACTERIZING IMAGING RADIATION RISK IN A POPULATION OF 8918 PATIENTS WITH RECURRENT IMAGING FOR A BETTER EFFECTIVE DOSE Article Open access 14 March 2024 THE DOSE–RESPONSE
CHARACTERISTICS OF FOUR NTCP MODELS: USING A NOVEL CT-BASED RADIOMIC METHOD TO QUANTIFY RADIATION-INDUCED LUNG DENSITY CHANGES Article Open access 29 June 2020 INTRODUCTION The Euratom
council directive (_2013/59_/_Euratom_) emphasizes the need for patient radiation dose monitoring in clinical routine1. Computed tomography (CT) is indispensable for contemporary patient
care, but many studies suggest a relation between low dose protracted radiation exposure and an increased incidence of malignancy based on the linear Non-threshold Dose-Response Model2.
Therefore, detailed knowledge of radiation exposure from CT examinations is crucial for radiology healthcare professionals in clinical routine to detect and address increased risks of
malignancy. Widely used parameters for radiation exposure assessment like the volumetric CT dose index (CTDIvol) and the Dose Length Product (DLP) characterize scanner radiation output, but
are unable to take individual patient characteristics into account3. Conversion to effective dose (EDDLP) is feasible using population-based conversion factors (k) that take the averaged
radiosensitivity in defined anatomic regions into account4. A variety of different k-factors are recommended in the literature for different volumes (e.g. head, thorax, abdomen, pelvis) and
different CT-scanners5,6. These k-factors are mostly derived from phantom models that try to represent average patient anatomy in western populations, but are unable to respect individual
anatomy like missing organs due to aplasia or resection, organ hypo- or hypertrophy, skeletal deformations and metal implants. Nevertheless, these programs, like e.g. the National Cancer
Institute (NCI) dosimetry system for CT, can be considered as current academic reference (EDNCI)7. However, the routinely performed calculation of EDDLP is imprecise and the degree of
difference to the real ED in each individual patient is unknown. Risk estimates that are calculated on these imprecise data could then easily be misinterpreted. Therefore, conventionally
calculated EDDLP can be used for comparison between different CT-scanners or different examination protocols, but should not be used for comparison between different patient collectives or
for radiation risk estimation8,9. Stochastic estimation of radiation exposure from CT is feasible by Monte Carlo (MC) simulations. Romanyukha _et al_. used computational phantoms and
MC-simulations to calculate body size-specific conversion factors from regression curves10. Huda _et al_. were able to show that this phantom based technique is also feasible to derive organ
doses and to use these for cancer risk estimation11. Only few studies with small numbers of mostly pediatric patients used MC simulations for individual dose and risk estimation per
patient, probably due to the high computational power needed12,13. The main drawback of MC calculations is that they are limited to the reconstructed body volume. Therefore, overexposure in
z-axis and scattered radiation to the organs outside the directly exposed volume cannot be taken into account. Detailed information about biological effects of low-level ionizing radiation
is available from the National Academy of Sciences report number seven about _Health Risks from Exposure to Low Levels of Ionizing Radiation_ (BEIR VII). The authors of BEIR VII report
follow the linear Non-threshold Dose-Response Model and presume that the risk of cancer incidence and mortality due to medical examinations are mainly based on low-level ionizing radiation
dose, age and gender14. The aim of this study was to provide individual calculations of ED in whole-body exposure derived from MC calculations (EDMC) and to compare them with the
conventional method (EDDLP) and the academic reference (EDNCI) following an equivalence hypothesis in a heterogeneous study collective. Furthermore, cancer risk estimates that are based on
EDMC should allow to evaluate the impact of patient characteristics on individual risk of malignancy. Whole body CT from combined Positron Emission Tomography (PET)-CT examinations were
exclusively selected in order to minimize the indeterminate radiation dose to body parts outside the imaging volume that cannot be assessed by MC. METHODS STUDY DESIGN Twenty-two patients
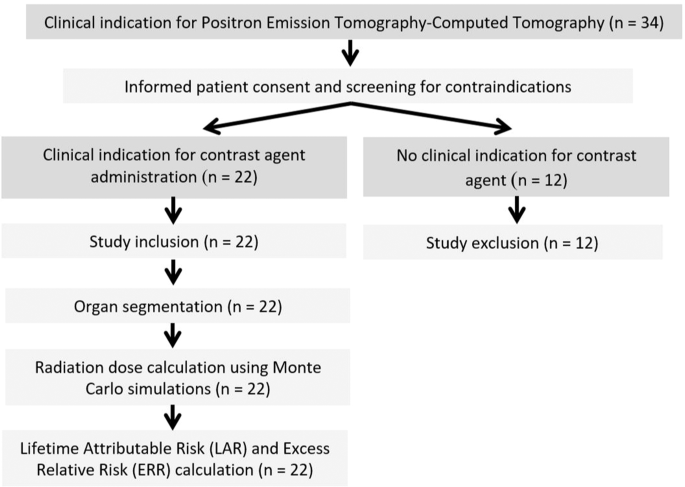
from a collective of 34 consecutive patients, with clinical indication for whole body PET-CT, were retrospectively included (Fig. 1). Inclusion criteria were the complete and artifact-free
coverage of the scan volume, sufficient intra-venous contrast injection and full availability of individual patient data including age, height, weight and body mass index (BMI). Exclusion
criteria were severe artifacts (n = 0) and examinations without contrast injection (n = 12). Clinical indications were infectious diseases (n = 8), bronchial carcinoma (n = 6), head and neck
cancer (n = 3), cancer of unknown primary (n = 2), malignant melanoma (n = 2) and retroperitoneal fibrosis (n = 1). Written informed consent was obtained and archived for each patient. The
study was approved by the local ethics committee of the Friedrich-Alexander University Erlangen-Nuremberg and complied with the Declaration of Helsinki. IMAGE ACQUISITION All examinations
were performed with continuous volume acquisition using a Siemens Biograph True Point 64 PET-CT (Siemens Healthcare GmbH, Forchheim, Germany). Indication, supervision and image analysis were
performed by senior physicians with extensive experience in PET-CT (>6 years). CT was performed from head to mid-femur by a single spiral acquisition in a cranio-caudal direction using a
tube voltage of 120 kV, anatomic tube current modulation with a reference tube-current time product of 170 mAs, pitch 0.8, gantry rotation time 0.5 seconds and 64 × 0.6 mm slice acquisition
by 32 × 0.6 mm detector collimation and z-axis flying focal spot double sampling15. Unlike in diagnostic CT, where different body parts (e.g. head/neck and thorax/abdomen) are often
examined in multiple contrast phases and different positions, PET-CT uses single spiral acquisitions with only one single bolus injection allowing for complete coverage of all body organs
without overlapping of anatomic regions. Therefore, the effect of scattered radiation to all radiosensitive tissues can be taken into account by MC calculations and the effect of overranging
in the beginning and end of the spiral acquisition can be neglected16. All patients received body weight adapted intravenous injection of 18F-fluorodeoxyglucose (3 MBq/kg). After an average
resting period of 91 (±25.6) minutes iodine containing contrast agent (Ultravist 370®, Bayer-Schering Healthcare, Berlin, Germany) was mechanically administered into an antebrachial cannula
using a power injector (Accutron CT-D, Medtron AG, Saarbruecken, Germany), and the single spiral CT acquisition was started with a fixed delay of 70 s. The PET acquisitions were run
subsequently in 7–8 steps, depending on patient length and exam volumes. For this study full field of view (500 × 500 mm) images were reconstructed using a smooth filtered back projection
kernel (B31), slice thickness 5.0 mm and increment 5.0 mm. The resulting voxel size was 0.98 × 0.98 × 5.00 mm. CONVENTIONAL CALCULATION OF EFFECTIVE DOSE Tube current time product, CTDIvol
and DLP were recorded for each examination from the PET-CT scan protocol as provided by the scanner. Details about their definition and calculation are reported elsewhere in literature6. The
main drawback of these monitoring techniques is that they are unable to provide information about the biological impact of radiation exposure and should only be taken as an index of
radiation output by the CT system for comparative purposes9,17. The biologically relevant effective dose (EDDLP) was calculated for each examination by multiplication of DLP and the
region-specific conversion factor. For this study kBody (0.015 mSv/mGy∙cm) was used as recommended by the American Association of Physicists in Medicine6. Dose exposition related to
18F-fluorodeoxyglucose (FDG)-PET were not considered for ED calculation. PHANTOM BASED CALCULATION OF EFFECTIVE DOSE Academic reference ED was calculated using a dedicated CT dosimetry
software tool provided by the National Cancer Institute (NCI-CT)7, which combines reference phantoms provided by the International Commission on Radiological Protection (ICRP) and MC
simulations of a reference CT scanner (Somatom Sensation 16, Siemens Healthcare GmbH, Forchheim, Germany). Individual examination parameters, gender, age, height and body weight were used as
input for this mathematical phantom based calculation (EDNCI). Dedicated descriptions about computational methods of this software are available in the literature7. ORGAN SEGMENTATION All
radiosensitive organs or tissues including all remainders, which are mentioned in IRCP report 103 were segmented using a dedicated software package (ITK-SNAP 1.8, Penn Image Computing and
Science Laboratory, Philadelphia, USA). Semi-automatic threshold segmentation with manual corrections was used for the lungs, cortical bone, bone marrow, liver, spleen, brain, heart, muscle
and skin. All other organs or tissues were segmented manually in a slice per slice fashion. Therefore, missing organs due to aplasia or resection, organ hypo- or hypertrophy, skeletal
deformations and metal implants were considered. For hollow organs, only voxels contributing to the wall were considered to contain radiosensitive tissues. Segmentation of lymphatic tissues
was restricted to visible lymphatic nodes (Fig. 2). All segmentations were performed by a specially trained radiology resident (4 years experience) and reviewed from an experienced
board-certified radiologist (9 years experience). MONTE CARLO BASED CALCULATION OF EFFECTIVE DOSE Extensive mathematical models in simulation techniques can be utilized to calculate dose
distribution for each voxel of CT scan volumes using the attenuation values and geometry from DICOM datasets as input18,19. All MC dose simulations were carried out using the software
package ImpactMC (VAMP GmbH, Erlangen, Germany). Software details and information concerning the Monte Carlo calculation algorithms are reported elsewhere18,20. Multiplication of the
resulting relative dose values per voxel with air kerma provides the absorbed dose for each voxel. The air kerma is a scanner specific value, which describes the kinetic energy transferred
in air dependent on geometry and tube settings. It can be considered as measurement of x-ray beam intensity without object, which was 18.3 mGy for this study. The averaged absorbed dose over
an organ volume, defined from segmentations, provides the absorbed organ dose. Equivalent organ dose was calculated by multiplication with the radiation weighting factor for photons (WR =
1) and used for subsequent effective organ dose calculations (EDOrgan) by multiplication with the dedicated tissue weighting factor (WT) as recommended in ICRP report 10321. The sum of all
effective organ doses provides EDMC for each individual (Fig. 3). The relative contribution of the respective EDOrgan to the EDMC was calculated following Eq. 1:
$${{\rm{C}}}_{{\rm{Organ}}}={{\rm{ED}}}_{{\rm{Organ}}}/{{\rm{ED}}}_{{\rm{MC}}}$$ (1) Individual conversion factors (kMC) were calculated using Eq. 2 to illustrate the differences compared to
the conventional calculation method using DLP and kbody. $${{\rm{k}}}_{{\rm{MC}}}={{\rm{ED}}}_{{\rm{MC}}}/{\rm{DLP}}$$ (2) RADIATION RISK ASSESSMENT Lifetime Attributable Risk (LAR) is
defined as risk of disease of an exposed cohort in comparison to a non-exposed cohort. Calculations of LAR for cancer mortality were based on report VII about Biologic Effects of Ionizing
Radiation (BEIR VII). The BEIRV VII report refers to an individual radiation dose exposure of 100 mGy. Assuming a linear risk distribution between decennium age intervals LARMC was derived
from EDMC by linear interpolation between the younger (Ny) and older (NO) cohort as shown in equation 3 using patient age (Ap) and the age of the younger cohort (Ay) as input values22.
Equation 3: Calculation of individual LAR $$LA{R}_{MC}=\left({N}_{y}-\left(({N}_{y}-{{\rm{N}}}_{0})\ast \frac{{A}_{p}-{A}_{Y}}{10\,{\rm{years}}}\right)\right)\ast
\frac{E{D}_{Organ}}{100\,{\rm{mGy}}}$$ The Excess Relative Risk (ERRMC), as a measurement of the exceeding risk of an exposed person compared to a non-exposed person, was calculated using
the solid cancer mortality in the United States as baseline (female: 17500/100000; male: 22100/100000) using equation 423. Equation 4: Calculation of individual ERR
$$ER{R}_{MC}=LA{R}_{MC}/LA{R}_{baseline}$$ PAIRWISE PATIENT COMPARISON Because of the rather small patient collective in this study, mainly due to the extensive effort needed for
segmentation of all radiosensitive tissues, pairwise patient comparisons were selected to highlight the influence of individual patient characteristics on the radiation dose parameters and
radiation risk estimation. Matching patient pairs, each with two comparable values and one variable parameter, were found for the parameters age, sex and BMI. STATISTICAL ANALYSIS All
statistical analyses were performed using the software package SPSS Statistics Version 21 (IBM, Somers, NY, USA). Normal distribution of the data was tested by Kolmogorov-Smirnov and
Shapiro-Wilk test. Normally distributed data is presented as mean ± standard deviation. Median and range are provided if no normal distribution was assumed. Illustration is provided as
Bland-Altman plots. Spearman’s rank order test was used to test for correlations between tube current, BMI, EDDLP and EDMC. The significance level was defined as p < 0.05. RESULTS
DEMOGRAPHICS OF THE STUDY COLLECTIVE Five out of 22 patients (23%) were female and 17 male (77%). The mean age was 57.3 ± 14.3 years and the mean BMI was 26.0 ± 5.9 kg/m². Three patients
(13.6%) were younger than 40 years. The female patient group was younger (53.6 ± 17.6 years) compared to the male patient group (58.4 ± 13.6 years). Female patients had a lower mean BMI
(21.2 ± 2.5 kg/m²) compared to male patients (27.4 ± 5.88 kg/m2). From the male subgroup 9 patients (52.9%) suffered from overweight (BMI > 25), and three patients (17.6%) had severe
overweight (BMI > 30). One female and one male patient were considered as underweight (BMI < 19). No overweight patient was found in the female subgroup. Detailed patient
characteristics are shown in Table 1. ORGAN SPECIFIC RADIATION DOSE EXPOSURE Mean equivalent organ dose was 13.0 ± 3.5 mGy. Highest equivalent organ dose values were found in high
attenuating structures such as the cortical bone and the thyroid gland. Lowest values were found in profound organs like the extra-thoracic (ET) respiratory region and the uterus. Many
superficial organs like muscles (11.5 ± 2.0 mGy), breast tissue (10.9 ± 0.8 mGy) and salivary glands (14.3 ± 2.8 mGy) also had rather low equivalent organ dose values. Despite its profound
position well perfused kidneys had very high equivalent organ dose values (17.0 ± 2.1 mGy). Effective organ dose was mainly influenced by WT, nicely demonstrated by the thyroid gland. It has
the second highest mean equivalent organ dose (21.2 ± 5.4 mGy), but is only ranked 7th highest effective organ dose (8% of total organ dose distribution) because of the rather low WT
(0.04). In contrast, the lungs ranked only 7th in equivalent organ dose (13.4 ± 1.9 mGy), but received highest effective organ dose levels in female and male patients (12% and 14% of the
total EDMC) due to high WT (0.12). Colon, lungs and stomach contributed to more than 50% of the total EDMC in male patients and to 48% in female patients (Fig. 4). Differences between WT and
the relative contribution of the effective organ dose to whole body EDMC (COrgan) reflect the influence of individual radiation dose distribution on each organ dose. Highest positive
differences between WT and COrgan were found for the bone surface (♀: +102.1%; ♂: +134.8%), the thyroid gland (♀: +89.1%; ♂: +78.1%) and the kidneys (♀: + 49.4%; ♂: +47.1%). The male gonads
also had substantially higher COrgan than expected from its rather low WT ( + 38.2%), but the female gonads had the highest negative difference of all organs (−21.8%). Other organs with high
negative differences were the adrenal glands (♀: −18.3%; ♂: −11.8%). Detailed organ dose information is shown in Table 2 and organ-based LAR of cancer mortality is provided for all organs
listed in BEIR VII. Considering the similar effective organ doses of the lung (1.48 ± 0.15 mSv) and the breast (1.30 ± 0.09 mSv) the LAR for pulmonary malignant disease (13.25 ±
4.24/100.000) was remarkably higher compared to the LAR for breast cancer mortality (2.46 ± 1.62/100.000). EFFECTIVE DOSE AND INDIVIDUAL RADIATION RISK ASSESSMENT EDDLP (13.2 ± 4.52 mSv) was
higher than EDMC (11.6 ± 1.47 mSv) and EDNCI (9.8 ± 2.1 mSv). Particularly high differences between EDMC and EDDLP were found for patients with relatively high or low radiation dose
exposure. Mean difference between both methods was −1.7 mSv (Fig. 5a). The same tendency was found for the comparison of EDNCI and EDDLP but with higher mean negative differences −3.4 mSv
(Fig. 5b). A comparison between the three calculation methods is illustrated as boxplot (Fig. 6). The range of radiation dose was substantially smaller in both advanced methods (EDMC: 5.6
mSv, EDNCI: 10.2 mSv) compared to the conventional technique (EDDLP: 19.3 mSv). Effective mAs and DLP linearly increased with higher BMI in Spearman’s rank order test (rs = 0.961 and 0.949).
This effect should be mainly due to the anatomy-based tube current modulation algorithm. Therefore, also BMI and EDDLP had a high correlation coefficient (rs = 0.949). The correlation
between EDMC and BMI (rs = 0.644) was less and differences between EDDLP and the advanced techniques was especially high in over- and underweight patients. For example, the highest EDDLP of
26.3 mSv was found in a 77-year-old man suffering from adiposity grade 3 (BMI 40.0 kg/m²) while EDNCI (17.3 mSv) and EDMC (13.9 mSv) were substantially lower (34% and 47% less). Lowest EDDLP
of 7.0 mSv was calculated for a 30-year-old underweight man (BMI 16.8 kg/m²), which was close to EDNCI (7.1 mSv), but substantially lower than EDMC (8.5 mSv, 21% higher). Consequently, the
individually calculated conversion factors (kMC) have a range from 53% to 140% when referred to kBody from literature. The mean kMC (0.014 ± 0.004 mSv/mGy cm) approximately reflects the
established value (0.015 mSv/mGy cm), which therefore seems to be suitable for regular weight patients. However, only one fourth of the patients in this study (n = 6, 27.2%) had less than
10% difference between kMC (0.0135–0.0165 mSv/mGy cm) and kBody (mean BMI 22.95 ± 1.58, range 20.7–25.0 kg/m²). The values of LAR were especially high in young female patients. The highest
value (60/100.000) was calculated for a 30-year-old, normal weight woman (BMI 21.6 kg/m²). In contrast, low LAR values were calculated for older, predominantly male patients. The lowest
value (23/100.000) was found for a 76-year-old, overweight man (BMI 29.8 kg/m²). Extent of EDMC, EDDLP and LAR often differed considerably when compared between young and old or underweight
and obese patients, while the interaction between these individual parameters was rather difficult to predict. Detailed results of radiation dose and risk calculations are provided in Table
3. PAIRWISE PATIENT COMPARISON Two male patients with matching constitution (BMI 24 vs. 23 kg/m²) but different age (36 versus 67 years) had comparable DLP (783 vs. 724 mGy · cm, −7.5%),
EDDLP (11.8 vs. 10.9 mSv, −7.5%), EDNCI (9.4 vs. 9.0 mSv, −4.3%) and EDMC (10.9 vs 10.0 mSv, −8.4%). Nevertheless, calculations for LAR of cancer mortality (41.3 vs. 27.1/100.000; −34.4%)
and ERR (0.19 vs. 0.12%, −36.8%) differed considerably. The young age seems to be the decisive factor among all the considered factors, accounting for about 50% higher risk estimates (Fig.
7a). Two male patients with matching age (76 and 77 years) but different BMI (29.7 vs. 40.0 kg/m², +34.7%) were exposed to considerably different DLP (998 vs. 1751 mGy·cm, +75.4%) based on
anatomic tube current modulation. Therefore, EDDLP calculations provided very high values for the obese patient (15.0 vs. 26.3 mSv, +75.5%). Dose differences calculated with EDNCI (10.5 vs.
17.3 mSv, +64.5%) were also considerably high, while EDMC values ware nearly comparable (11.9 vs. 13.8 mSv; +15.9%). Differences in LARMC (22.8 vs. 25.1/100.000; +9.6%) and ERR (0.10 vs
0.11%; +10.0%) were even less (Fig. 7b). BMI seems to have only a minor influence on effective dose and risk estimation, while conventional methods would have resulted in an overestimation
of almost 100%. Two patients with matching age (56 and 54 years) and BMI (21.5 vs 21.0 kg/m²) but different sex had similar DLP (634 vs. 630 mGy·cm; +0.63%). ED was the same using the
conventional method in both patients (EDDLP = 9.5 mSv) and almost the same with the NCI method (EDNCI = 8.7 vs 9.0 mSv; −3.3%). EDMC calculation was slightly higher for the female patient
(12.3 vs. 10.3 mSv; +19.4%), but LAR (53.1 vs. 35.4/100000; +50.2%) and ERR (0.3% vs. 0.16%; +87.5%) were substantially higher. Sex seems to have a dominant impact on the risk estimation.
Moreover, the unfavorable combination of age and sex leads to the highest values in the entire study collective, despite average ED values (Fig. 7c). DISCUSSION Individual radiation dose
assessment and risk calculation is feasible by image based Monte Carlo simulations and organ segmentations in an adult clinical routine collective that underwent full body exposure in a
single spiral acquisition. This is in good agreement with the findings of Li _et al_. and Tian _et al_. who evaluated comparable techniques for radiation dose estimation in small pediatric
collectives (n = 2 and n = 42)16,24. A comparable approach for cancer risk estimation in adults based on anthropomorphic phantoms was described by Huda _et al_.11. In contrast to these prior
studies, we provide a combination of individual radiation dose analysis, voxel-based organ segmentation and cancer risk estimation in direct comparison to such phantom based estimation and
the DLP method. The need for such an advanced dose monitoring method, due to several uncertainties with the conventional techniques, has been raised elsewhere in literature20,25. In our
study high equivalent organ doses were strongly related to radiodensity (e.g. bone surface and high contrast medium uptake in the kidneys, the thyroid gland and the extra-thoracic
respiratory region), which seems to be a much stronger predictor for equivalent organ dose than anatomical organ position. This is in good agreement with the contrast media related increase
of DNA double-strand break foci after radiation exposure reported in a prior study for CT26. WT is the strongest predictor of effective organ dose. For example, the thyroid gland and the
kidneys have the second and third highest equivalent organ dose (21.2 ± 3.2 mSv and 17.0 ± 2.1 mSv). However, due to low tissue weighting factors (WT = 0.04 and 0.0092) effective organ dose
of the thyroid gland and the kidneys were only sixth and twelfth highest. Our results confirm that individual patient characteristics have a considerable impact on radiation dose
calculation, which is underrepresented by the conventional method. The calculated error in our adult collective (−48 to 39%) is comparable to the previously published study results for
children (−63 to 28%)19. Individually calculated kMC from our study reveals that the routinely used kbody from literature can be applied to larger clinical collectives, but are limited in
their value for individual risk assessment6. The DLP-method slightly overestimates the effective dose in general, but it seems to be appropriate in regular weight male patients. However,
especially in female and underweight patients, underestimation of the effective dose may be critical for risk perception in the clinical setting. Moreover, its overestimation in obese
patients may lead to restrained use of high exposure parameters, and then to poor or insufficient image quality. Individual anatomic characteristics (e.g. missing organs due to aplasia or
resection, organ hypo- or hypertrophy, skeletal deformations, metal implants) may lead to considerable changes of radiation dose distribution and consequently of individual risk. Even
phantom based estimation methods, like EDNCI in this study, are unable to take these individual properties into account7. Bland-Altman analysis demonstrated that these phantom based
estimates have the tendency to underestimate ED in general, while the effect of only moderate increase of ED in obese patients despite the very high energy exposure is confirmed. The
influence of personal risk profiles on cancer risk estimates are not yet represented in standard radiation dose reporting systems. It can further increase the error of perceived and true
risk of radiation dose from CT examinations. The combination of individual dose distributions and risk assessment has been presented for a few other examinations in literature, such as
absorptiometry and spine radiographs, but not for spiral CT examinations27. Pairwise patient comparisons provide a comprehensive overview of the extent of error with regard to age, BMI and
sex. Especially younger patients are prone to higher LAR and ERR since more active cell division and longer life expectancy after radiation exposure is presumed28. In our example the ERR of
a patient in his mid-thirties was 1.6-fold the ERR of a patient in his mid-sixties and the ERR for a female patient in her mid-fifties was almost doubled compared to a matching male patient.
The increasing tube current by anatomy-based modulation in obese patients seems to be of less importance to the patients’ cancer risk. CTDI and EDDLP were 1.8-fold higher for a high-grade
obese patient compared to an overweight patient with matching age and sex, while the ERR was only 10% higher. Therefore, future dose assessment strategies should not only focus on absolute
dose values. Furthermore, reporting LAR or ERR seems to be much more appropriate in clinical routine and a more comprehensible value for the patient-physician interaction. Some limitations
have to be considered while interpreting this study. First, the study population consisted of a heterogeneous, small and retrospectively selected patient collective with an imbalanced ratio
of female to male patients. The small number of patients is mainly due to the high computing power required for MC calculations and the work intense manual organ segmentation that we used
for this study. We estimate that the increasing computing power and automated organ segmentations by the application of artificial intelligence could overcome this limitation in the near
future29,30. Second, only full body dose exposure is reported in this study. This avoids indeterminate scattered radiation to radiosensitive structures and over-ranging effects, but limits
the findings to this clinically rather rare indication. Further evaluations for limited examination volumes could become feasible with retrospective simulations from these data in larger
collectives by future studies. Third, all examinations were conducted on the same scanner. The findings of this study can therefore not automatically be transferred to other CT systems5,24.
Forth, all tissue weighting factors that are provided in literature so far are averaged for sex and age, which may probably limit their applicability for patient specific risk estimation.
Fifth, the linear Non-threshold Dose-Response Model itself is discussed controversially among experts for diagnostic dose levels31,32. Sixth, LAR and ERR calculations in this study are only
related to low dose radiation exposure. They illustrate the individual radiation dose related risk to develop cancer. Obviously, the overall individual cancer risk for a primary or even a
secondary cancer and also the life expectancy is potentially much more influenced by age, genetic make-up, efficiency of DNA damage repair, therapy-related adverse effects and many other
influencing factors. The importance of patient specific dose surveillance is illustrated by this study. EDDLP can be used for radiation dose assessments in larger collectives, but individual
considerations require advanced techniques like EDMC. Conventional methods tend to underestimate radiation dose in underweight and female patients. Therefore, these patients have to be
evaluated with special care. The influence of young patient age and female gender on cancer risk estimates is very high. Thus, radiation dose assessment should not only provide whole body or
organ dose measurements but also individual risk calculations, which could be included in future surveillance programs. DATA AVAILABILITY The datasets generated during and analyzed during
this study are available from the corresponding author on reasonable request. REFERENCES * EC. COUNCIL DIRECTIVE 2013/59/EURATOM of 5 December 2013 laying down basic safety standards for
protection against the dangers arising from exposure to ionising radiation, a. r. D. * Laurier, D. _et al_. The International Nuclear Workers Study (Inworks): A Collaborative Epidemiological
Study to Improve Knowledge About Health Effects of Protracted Low-Dose Exposure. _Radiation protection dosimetry_ https://doi.org/10.1093/rpd/ncw314 (2016). Article Google Scholar * Huda,
W. & Mettler, F. A. Volume CT dose index and dose-length product displayed during CT: what good are they? _Radiology_ 258, 236–242, https://doi.org/10.1148/radiol.10100297 (2011).
Article PubMed Google Scholar * Blackwell, C. R. & McCullough, E. C. A chamber and electrometer calibration factor as determined by each of the five AAPM accredited dosimetry
calibration laboratories. _Medical physics_ 19, 207–208, https://doi.org/10.1118/1.596880 (1992). Article ADS CAS PubMed Google Scholar * Hill, K. D. _et al_. Radiation Safety in
Children With Congenital and Acquired Heart Disease: A Scientific Position Statement on Multimodality Dose Optimization From the Image Gently Alliance. _JACC. Cardiovascular imaging_ 10,
797–818, https://doi.org/10.1016/j.jcmg.2017.04.003 (2017). Article PubMed PubMed Central Google Scholar * AAPM. The Measurement, reporting, and management of radiation dose in CT,.
_AAPM Report No. 96_ (2008). * Lee, C., Kim, K. P., Bolch, W. E., Moroz, B. E. & Folio, L. NCICT: a computational solution to estimate organ doses for pediatric and adult patients
undergoing CT scans. _Journal of radiological protection: official journal of the Society for Radiological Protection_ 35, 891–909, https://doi.org/10.1088/0952-4746/35/4/891 (2015). Article
ADS CAS Google Scholar * Martin, C. J. Effective dose: how should it be applied to medical exposures? _The British journal of radiology_ 80, 639–647,
https://doi.org/10.1259/bjr/25922439 (2007). Article CAS PubMed Google Scholar * McCollough, C. H., Christner, J. A. & Kofler, J. M. How effective is effective dose as a predictor of
radiation risk? _AJR. American journal of roentgenology_ 194, 890–896, https://doi.org/10.2214/AJR.09.4179 (2010). Article PubMed Google Scholar * Romanyukha, A., Folio, L., Lamart, S.,
Simon, S. L. & Lee, C. Body Size-Specific Effective Dose Conversion Coefficients for Ct Scans. _Radiation protection dosimetry_ 172, 428–437, https://doi.org/10.1093/rpd/ncv511 (2016).
Article CAS PubMed Google Scholar * Huda, W. & He, W. Estimating cancer risks to adults undergoing body CT examinations. _Radiation protection dosimetry_ 150, 168–179,
https://doi.org/10.1093/rpd/ncr376 (2012). Article PubMed Google Scholar * Li, X. _et al_. Patient-specific dose estimation for pediatric chest CT. _Medical physics_ 35, 5821–5828,
https://doi.org/10.1118/1.3026593 (2008). Article ADS PubMed PubMed Central Google Scholar * Kost, S. D. _et al_. Patient-specific dose calculations for pediatric CT of the chest,
abdomen and pelvis. _Pediatric radiology_ 45, 1771–1780, https://doi.org/10.1007/s00247-015-3400-2 (2015). Article PubMed PubMed Central Google Scholar * In Health Effects of Exposure to
Low Levels of Ionizing Radiations: Time for Reassessment? (1998). * Kyriakou, Y., Kachelriess, M., Knaup, M., Krause, J. U. & Kalender, W. A. Impact of the z-flying focal spot on
resolution and artifact behavior for a 64-slice spiral CT scanner. _European radiology_ 16, 1206–1215, https://doi.org/10.1007/s00330-005-0118-9 (2006). Article PubMed Google Scholar *
Li, X. _et al_. Patient-specific radiation dose and cancer risk estimation in CT: part II. Application to patients. _Medical physics_ 38, 408–419, https://doi.org/10.1118/1.3515864 (2011).
Article ADS PubMed Google Scholar * McCollough, C. H. _et al_. CT dose index and patient dose: they are not the same thing. _Radiology_ 259, 311–316,
https://doi.org/10.1148/radiol.11101800 (2011). Article PubMed PubMed Central Google Scholar * Deak, P., van Straten, M., Shrimpton, P. C., Zankl, M. & Kalender, W. A. Validation of
a Monte Carlo tool for patient-specific dose simulations in multi-slice computed tomography. _European radiology_ 18, 759–772, https://doi.org/10.1007/s00330-007-0815-7 (2008). Article
PubMed Google Scholar * DeMarco, J. J. _et al_. A Monte Carlo based method to estimate radiation dose from multidetector CT (MDCT): cylindrical and anthropomorphic phantoms. _Physics in
medicine and biology_ 50, 3989–4004, https://doi.org/10.1088/0031-9155/50/17/005 (2005). Article ADS CAS PubMed Google Scholar * Jarry, G., DeMarco, J. J., Beifuss, U., Cagnon, C. H.
& McNitt-Gray, M. F. A Monte Carlo-based method to estimate radiation dose from spiral CT: from phantom testing to patient-specific models. _Physics in medicine and biology_ 48,
2645–2663 (2003). Article ADS CAS Google Scholar * The 2007 Recommendations of the International Commission on Radiological Protection. ICRP publication 103. _Annals of the ICRP_ 37,
1–332, https://doi.org/10.1016/j.icrp.2007.10.003 (2007). * Rampinelli, C. _et al_. Exposure to low dose computed tomography for lung cancer screening and risk of cancer: secondary analysis
of trial data and risk-benefit analysis. _Bmj_ 356, j347, https://doi.org/10.1136/bmj.j347 (2017). Article PubMed PubMed Central Google Scholar * Lee, W. C. Excess relative risk as an
effect measure in case-control studies of rare diseases. _PloS one_ 10, e0121141, https://doi.org/10.1371/journal.pone.0121141 (2014). Article CAS PubMed Google Scholar * Tian, X. _et
al_. Pediatric chest and abdominopelvic CT: organ dose estimation based on 42 patient models. _Radiology_ 270, 535–547, https://doi.org/10.1148/radiol.13122617 (2014). Article PubMed
PubMed Central Google Scholar * Jansen, J. T. & Shrimpton, P. C. Development of Monte Carlo simulations to provide scanner-specific organ dose coefficients for contemporary CT.
_Physics in medicine and biology_ 61, 5356–5377, https://doi.org/10.1088/0031-9155/61/14/5356 (2016). Article ADS CAS PubMed Google Scholar * Grudzenski, S., Kuefner, M. A., Heckmann,
M. B., Uder, M. & Lobrich, M. Contrast medium-enhanced radiation damage caused by CT examinations. _Radiology_ 253, 706–714, https://doi.org/10.1148/radiol.2533090468 (2009). Article
PubMed Google Scholar * Law, M. _et al_. Cumulative Effective Dose and Cancer Risk of Pediatric Population in Repetitive Whole-Body Scan Using Dual-Energy X-Ray Absorptiometry. _Journal of
clinical densitometry: the official journal of the International Society for Clinical Densitometry_, https://doi.org/10.1016/j.jocd.2017.09.005 (2017). * Kalra, M. K., Sodickson, A. D.
& Mayo-Smith, W. W. CT Radiation: Key Concepts for Gentle and Wise Use. _Radiographics: a review publication of the Radiological Society of North America, Inc_ 35, 1706–1721,
https://doi.org/10.1148/rg.2015150118 (2015). Article Google Scholar * McBee, M. P. _et al_. Deep Learning in Radiology. _Academic radiology_, https://doi.org/10.1016/j.acra.2018.02.018
(2018). * Lee, H. _et al_. Pixel-Level Deep Segmentation: Artificial Intelligence Quantifies Muscle on Computed Tomography for Body Morphometric Analysis. _Journal of digital imaging_ 30,
487–498, https://doi.org/10.1007/s10278-017-9988-z (2017). Article PubMed PubMed Central Google Scholar * Shore, R. _et al_. Implications of recent epidemiologic studies for the linear
nonthreshold model and radiation protection. _Journal of radiological protection: official journal of the Society for Radiological Protection_, https://doi.org/10.1088/1361-6498/aad348
(2018). * Kase, K. R. Radiation protection principles of NCRP. _Health physics_ 87, 251–257 (2004). Article CAS Google Scholar Download references AUTHOR INFORMATION AUTHORS AND
AFFILIATIONS * Department of Radiology, University Hospital Erlangen, Erlangen, Germany Markus Kopp, Wolfgang Wuest, Michael Brand, Matthias Wetzl, Wolfram Nitsch, Michael Uder &
Matthias May * Institute of Medical Microbiology and Hygiene, University of Technology Dresden, Dresden, Germany Tobias Loewe * Department of Nuclear Medicine, University Hospital Erlangen,
Erlangen, Germany Daniela Schmidt & Michael Beck * Siemens Healthineers GmbH, Forchheim, Germany Bernhard Schmidt Authors * Markus Kopp View author publications You can also search for
this author inPubMed Google Scholar * Tobias Loewe View author publications You can also search for this author inPubMed Google Scholar * Wolfgang Wuest View author publications You can also
search for this author inPubMed Google Scholar * Michael Brand View author publications You can also search for this author inPubMed Google Scholar * Matthias Wetzl View author publications
You can also search for this author inPubMed Google Scholar * Wolfram Nitsch View author publications You can also search for this author inPubMed Google Scholar * Daniela Schmidt View
author publications You can also search for this author inPubMed Google Scholar * Michael Beck View author publications You can also search for this author inPubMed Google Scholar * Bernhard
Schmidt View author publications You can also search for this author inPubMed Google Scholar * Michael Uder View author publications You can also search for this author inPubMed Google
Scholar * Matthias May View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Contributed to the writing of the manuscript: M.K., M.M., T.L. and
W.W. Study design, measurements and statistical analysis: M.K., M.M., W.W., M.Br., M.W., T.L., W.N., B.S., D.S., M.Be. and M.U. All authors reviewed the manuscript. CORRESPONDING AUTHOR
Correspondence to Markus Kopp. ETHICS DECLARATIONS COMPETING INTERESTS M.K., M.M., W.W. and M.U. are members of the Siemens Speaker’s Bureau. B.S. is an employee of Siemens Healthineers
GmbH. M.W., T.L., M.Br., W.N., D.S. and M.Be. declare no competing financial interests. All authors declare no competing non-financial interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a
Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit
to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are
included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and
your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this
license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Kopp, M., Loewe, T., Wuest, W. _et al._ Individual Calculation of
Effective Dose and Risk of Malignancy Based on Monte Carlo Simulations after Whole Body Computed Tomography. _Sci Rep_ 10, 9475 (2020). https://doi.org/10.1038/s41598-020-66366-2 Download
citation * Received: 21 January 2019 * Accepted: 14 May 2020 * Published: 11 June 2020 * DOI: https://doi.org/10.1038/s41598-020-66366-2 SHARE THIS ARTICLE Anyone you share the following
link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature
SharedIt content-sharing initiative