Unprecedented enhancement of recombinant protein production in sugarcane culms using a combinatorial promoter stacking system
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Plants represent a safe and cost-effective platform for producing high-value proteins with pharmaceutical properties; however, the ability to accumulate these in commercially viable
quantities is challenging. Ideal crops to serve as biofactories would include low-input, fast-growing, high-biomass species such as sugarcane. The objective of this study was to develop an
efficient expression system to enable large-scale production of high-value recombinant proteins in sugarcane culms. Bovine lysozyme (BvLz) is a potent broad-spectrum antimicrobial enzyme
used in the food, cosmetics and agricultural industries. Here, we report a novel strategy to achieve high-level expression of recombinant proteins using a combinatorial stacked promoter
system. We demonstrate this by co-expressing _BvLz_ under the control of multiple constitutive and culm-regulated promoters on separate expression vectors and combinatorial plant
transformation. BvLz accumulation reached 1.4% of total soluble protein (TSP) (10.0 mg BvLz/kg culm mass) in stacked multiple promoter:_BvLz_ lines, compared to 0.07% of TSP (0.56 mg/kg) in
single promoter:_BvLz_ lines. BvLz accumulation was further boosted to 11.5% of TSP (82.5 mg/kg) through event stacking by re-transforming the stacked promoter:_BvLz_ lines with additional
_BvLz_ expression vectors. The protein accumulation achieved with the combinatorial promoter stacking expression system was stable in multiple vegetative propagations, demonstrating the
feasibility of using sugarcane as a biofactory for producing high-value proteins and bioproducts. SIMILAR CONTENT BEING VIEWED BY OTHERS IDENTIFICATION OF A SUGARCANE BACILLIFORM VIRUS
PROMOTER THAT IS ACTIVATED BY DROUGHT STRESS IN PLANTS Article Open access 26 March 2024 POPLAR TRANSFORMATION WITH VARIABLE EXPLANT SOURCES TO MAXIMIZE TRANSFORMATION EFFICIENCY Article
Open access 08 January 2025 ENERGY-EFFICIENT PRODUCTION OF VACCINE PROTEIN AGAINST PORCINE EDEMA DISEASE FROM TRANSGENIC LETTUCE (_LACTUCA SATIVA_ L.) Article Open access 24 September 2022
INTRODUCTION Recombinant proteins are currently being produced in cultured cell-based systems in mammals, microbes (bacteria and yeast), insects and plants, as well as in transgenic animals
(reviewed by Demain and Vaishnav)1. Transgenic plants constitute an attractive system for expression and production of a variety of proteins and biomolecules due to their efficient
eukaryotic protein synthesis, high scalability, relatively low production costs and environmental footprint2,3,4. However, selecting suitable hosts and expression vectors are key
considerations since protein accumulation is determined by expression levels. Important factors to consider when selecting a plant-based production platform include biomass yield per
hectare, recombinant protein yield per unit biomass, ease of transformation, scalability and safety5. Sugarcane (_Saccharum_ spp. hybrids), a key feedstock in the expanding bioeconomy as a
sugar and bioenergy crop6, is an ideal platform for recombinant protein production for several reasons: (1) It is a relatively fast growing tropical grass with the highly efficient C4
photosynthetic pathway, conferring high biomass production capacity with yields of up to 41.3 tons of biomass (harvested dry mass) per hectare per annum7,8; (2) it is highly efficient in
utilizing radiation, water and nutrients to produce a large biomass and hence a higher recombinant protein yield; (3) it is readily amenable to genetic engineering, with established
transformation and tissue regeneration techniques9,10; and (4) it has a low risk of out-crossing recombinant genes due to its primarily vegetative means of propagation; natural reproductive
propagation in many temperate and subtropical regions is rare due to its photoperiod sensitivity. Sugarcane was used as biofactory for the production of new biomolecules such as
bioplastics11,12,13,14,15, alternative sugars (sorbitol and isomaltulose)16,17,18, and recombinant proteins including the human cytokine granulocyte macrophage colony stimulating factor
GM-CSF19, canecystatins (cysteine protease inhibitors) CaneCP-1, CaneCP-2 and CaneCP-320,21,22, and the cellulolytic enzymes, endoglucanase and cellobiohydrolases I and II23,24. Accumulation
levels of these recombinant proteins ranged from 0.02 to 2.0% of total soluble protein (TSP) in leaves. However, very few attempts have so far been made to express recombinant proteins in
sugarcane culms (reporter proteins)25, which constitute the largest fraction of harvestable biomass and would be an ideal platform for production of bulk proteins. Bovine lysozyme (BvLz) is
more important industrially than other lysozymes because of its potent broad-spectrum antimicrobial activity26,27, especially against Gram-negative bacteria and fungi at concentrations as
low as 25 ppm, its sixfold higher chitinase activity than that of chicken lysozyme28, and its thermal stability and resistance to proteolysis29. BvLz, unlike other enzymes, possesses
biochemical properties that make it suitable for protein extraction and purification, such as stability over a broad pH range, thermal stability, resistance to proteolysis and convenient
quantification assays30,31. In this study, we demonstrate the feasibility of developing sugarcane as an expression platform for production and purification of recombinant proteins at high
levels, i.e. up to 11.5% of TSP (82.5 mg protein/kg culm mass). Multiple promoters (constitutive or culm-regulated) on separate expression vectors were stacked by combinatorial plant
transformation approach to boost production levels of recombinant _bovine lysozyme_ (_BvLz_), which was codon-optimized for expression in monocots. A double terminator or 3′ untranslated
region (UTR) was incorporated for improved transcript stability. Enzymatic activity and enzyme-linked immunosorbent assays (ELISA) of _BvLz_ transgenic sugarcane culm protein extracts and
clarified juice confirmed the presence of an intact and fully active BvLz enzyme, which accumulated in multiple vegetative generations at levels as high as 10.0 mg/kg (1.4% of TSP) in lines
co-expressing _BvLz_ from stacks of three or four different promoters on separate vectors, compared to 0.56 mg/kg (0.07% of TSP) in lines expressing _BvLz_ from a single promoter vector. We
further observed BvLz accumulation up to 82.5 mg/kg (11.5% of TSP) through event stacking by re-transforming the stacked promoter:_BvLz_ transgenic lines with additional _BvLz_ expression
vectors. RESULTS AND DISCUSSION THE COMBINATORIAL PROMOTER AND EVENT STACKING RESULT IN INCREASED RECOMBINANT PROTEIN PRODUCTION IN TRANSGENIC SUGARCANE CULMS A salient feature of
combinatorial transformation, a special case of co-transformation32, is that there is no theoretical limit to the number of expression vectors that can be co-transformed. To enable
high-levels of recombinant protein production in sugarcane culms, we developed a combinatorial promoter and event stacking system and demonstrated its application in producing a high-value
bovine lysozyme (BvLz) protein. This was facilitated by the availability of a set of constitutive and culm-regulated promoters previously isolated from sugarcane, in addition to the common
maize _ubiquitin 1_ promoter (pUbi)33. These include the culm-regulated promoters for _Sugarcane bacilliform virus_ (pSCBV21)34 and sugarcane _dirigent16_ (pSHDIR16) gene35, and the
constitutive promoters for sugarcane _proline-rich protein_ (pSHPRP)36 and _elongation factor 1α_ (pSHEF1α)36 genes. Furthermore, conditions for small-scale and large-scale extraction and
clarification of recombinant BvLz from sugarcane culm extracts and juice were optimized at our Pilot Plant and BioSeparation Facilities30,37. The essential design of the resulting new
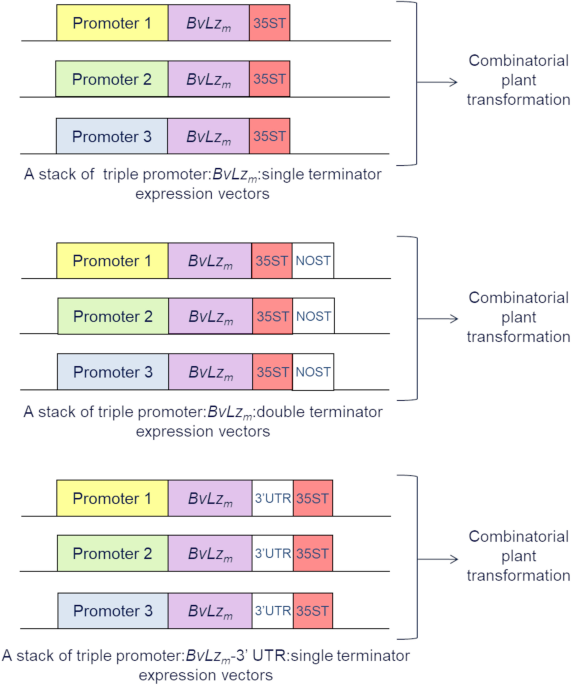
combinatorial promoter stacking system is illustrated in Fig. 1. The system consisted of co-expressing the codon-optimized _BvLz__m_, from a stack of multiple promoters on separate
expression vectors in sugarcane by combinatorial transformation. A double terminator, composed of the _Cauliflower mosaic virus_ (CaMV) 35S terminator (35ST) and the _Agrobacterium
tumefaciens_ nopaline synthase terminator (NOST), or the 3′UTR of _Sorghum mosaic virus_ (SrMV), was fused to the coding region of _BvLz__m_ to enhance transcript stability38,39 (Fig. 1). To
test the stacking promoter gene expression system, embryogenic calli (2 month-old) and leaf roll discs (12 day-old), prepared from several commercial sugarcane varieties were co-transformed
biolistically with the multiple promoter:_BvLz__m_ expression vectors, using the _bar_ gene (phosphinothricin acetyl transferase) as a selectable marker. Several independent transgenic
_BvLz__m_ lines, identified by Southern blot analysis (Fig. 2a; Supplementary Fig. S2), were generated from the combinatorial transformation of sugarcane with single, double, triple or
quadruple promoter:_BvLz__m_ expression vectors (Table 1). These represent: (1) 43 lines (114 plants) expressing _BvLz__m_ from a single promoter, (2) 10 lines (52 plants) expressing
_BvLz__m_ from a double promoter stack, (3) 24 lines (318 plants) expressing _BvLz__m_ from a triple promoter stack, and (4) 23 lines (76 plants) expressing _BvLz__m_ from a quadruple
promoter stack (Table 1). The integration and size of each respective _BvLz__m_ expression vector (promoter, _BvLz__m_, terminator and/or 3′UTR) in the single and stacked multiple
promoter:_BvLz__m_ lines were confirmed by Southern blot hybridization with a full-length _BvLz__m_ probe (Fig. 2a; Supplementary Fig. S2) and by PCR using primers encompassing each of the
different promoter:_BvLz__m_-terminator cassettes (Fig. 3; Supplementary Fig. S4, S5 and S6). All lines were analyzed for their _BvLz__m_ transcript levels by northern blot hybridization
(Fig. 2b; Supplementary Fig. S3) as well as for their BvLzm accumulation by ELISA (Table 1; Fig. 2c for ELISA). For representative lines, yield was also determined by an enzyme activity
assay and the results highly correlated with the ELISA data (R = 0.81–0.98; Supplementary Table S1). Furthermore, in general, a clear positive trend was observed between the _BvLz__m_ copy
number, the combinatorial promoter-_BvLz__m_ cassettes transformed and the BvLzm levels (Table 2; Fig. 2; Supplementary Fig. S2). For instance, quadruple and triple promoter:_BvLz__m_ lines
displayed a higher _BvLz__m_ copy number and yield than double and single promoter:_BvLz__m_ lines, as expected from co-transformation (Table 2; Fig. 2; Supplementary Fig. S2). Similarly,
the double promoter pUD:_BvLz__m_ lines had a higher _BvLz__m_ copy number and accumulation than single promoter pU:_BvLz__m_ lines (Table 2; Fig. 2; Supplementary Fig. S2). The BvLzm yield
from single promoter pUbi:_BvLz__m_ (pU:_BvLz__m_) lines varied from low (0.08–0.1 mg/kg; 6.7% of plants) to moderate (0.12–0.18 mg/kg; 40.0% of plants) and high (0.2–0.4 mg/kg; 53.3% of
plants) (Tables 1, 2). The BvLzm yield range was 0.08–0.4 mg/kg (0.01–0.06% of TSP), averaging 0.3 mg/kg (0.04% of TSP) ± 0.02 for the high expressers (Table 1). Other single
promoter:_BvLz__m_ lines harboring pSHDIR16, pSCBV21, pSHPRP or pSHEF1α showed similar trends, with a highest BvLzm yield of 0.56 mg/kg (0.08% of TSP) (Supplementary Table S2). Stacked
double promoter pUbi-SHDIR16:_BvLz__m_ (pUD:_BvLz__m_) lines displayed 1.8–6.3 fold higher BvLzm yield than single promoter pU:_BvLz__m_ lines, with a range of 0.5–0.7 mg/kg (0.07–0.1% of
TSP) (Table 1). The BvLzm yield was further enhanced to 2.0–8.6 fold in the stacked triple promoter:_BvLz__m_ lines, with levels ranging from 1.0 to 6.0 mg/kg (0.1–0.8% of TSP) (Table 1).
The majority (66.7%) of the stacked triple promoter pUbi-SHPRP-SHEF1α:_BvLz__m_ (pUPE:_BvLz__m_) lines had a BvLzm yield of 1.0–2.0 mg/kg (0.1–0.3% of TSP) with 20.0% at 2.2–3.2 mg/kg
(0.33–0.45% of TSP) and 13.3% at 3.5–4.7 mg/kg (0.5–0.7% of TSP) (Table 1). Replacing the constitutive SHPRP promoter with the culm-regulated SHDIR16 promoter in the stacked triple promoter
pUbi-SHDIR16-SHEF1α:_BvLz__m_ (pUDE:_BvLz__m_) lines boosted the BvLzm yield to 6.0 mg/kg (0.8% of TSP). Most of pUDE:_BvLz__m_ lines (62.0%) had a BvLzm yield of 2.2–3.2 mg/kg (0.33–0.45%of
TSP), with 27.0% at 1.5–2.0 mg/kg (0.2–0.3% of TSP), 4.5% at 3.5–4.7 mg/kg (0.5–0.7% of TSP) and 6.5% at 5.0–6.0 mg/kg (0.7–0.8% of TSP) (Table 1). Next, we checked if stacking another
promoter to produce quadruple promoter:_BvLz__m_ lines would be helpful. The BvLzm yield increased modestly in the stacked quadruple promoter pUbi-SHPRP-SCBV21-SHEF1α:_BvLz__m_
(pUPBE:_BvLz__m_) lines by 1.7–2.4 fold, compared to the stacked triple promoter:_BvLz__m_ lines. The highest enhancement was achieved when using a double terminator cassette, i.e. 10.0
mg/kg (1.4% of TSP) in pUPBE:_BvLz__m_:35STNOST lines (Table 1), and the 3′UTR of SrMV with the single 35S terminator, i.e. 6.3 mg/kg (0.9% of TSP) in pUPBE:_BvLz__m_:3′UTR35ST lines (Table
1). In fact, 24.1% of pUPBE:_BvLz__m_:35STNOST plants had a BvLzm yield of 6.0–10 mg/kg (0.8–1.4% of TSP), and 44.5% of pUPBE:_BvLz__m_:3′UTR35ST plants showed a BvLzm yield of 6.0–6.3 mg/kg
(0.8–0.9% of TSP) (Table 1). Lastly, we evaluated if event stacking can enhance the yields of the stacked quadruple promoter lines. Event stacking, also referred to as super transformation,
is a good alternative to hybridization/crossing, which is time-consuming and not a viable option in vegetatively-propagated crops like sugarcane. Stacked five promoter
pUbi-SHDIR16-SHEF1α-SHPRP-SCBV21:_BvLz__m_ (pUDEPB:_BvLz__m_) lines were generated through event stacking, by re-transforming bialaphos-resistant triple promoter pUDE:_BvLz__m_ lines with
two promoter:_BvLz__m_ expression vectors, pP:_BvLz__m_ and pB:_BvLz__m_ (Table 1) using the _neomycin phosphotransferase II_ as a selectable marker. The resulting pUDEPB:_BvLz__m_ lines
showed increased BvLzm accumulation, i.e. up to 82.5 mg/kg culm mass (11.5% of TSP) (Table 1). The majority (33.3%) of these lines exhibited BvLzm levels of 26.2–32.3 mg/kg (3.6–4.5% of
TSP), while 18.3% accumulated the highest BvLzm levels, i.e. 59.9–82.5 mg/kg (8.3–11.5% of TSP). The remaining 24.2% and 12.1% of the lines showed BvLzm levels of 15.9–21.1 mg/kg (2.2–2.9%
of TSP) and 11.0–12.4 mg/kg (1.5–1.7% of TSP), respectively (Table 1). Notably, BvLzm accumulation was highly enhanced in the new stacked five promoter pUDEPB:_BvLz__m_ lines by
7.3–13.8-fold, compared to the receiving stacked triple promoter pUDE:_BvLz__m_ lines. Together, these experiments demonstrate that high levels of recombinant BvLzm (up to 11.5% of TSP or
82.5 mg/kg) can be successfully produced in sugarcane culms using the combinatorial promoter and event stacking strategies. Previous studies utilized multiple plant species, tissue types,
and expression systems for recombinant protein production40,41. Majority of them used transient _Agrobacterium_- and viral vector-based approaches in _Nicotiana benthamiana_ or _N.
tabacum_42,43,44,45,46. While the transient systems are viable approaches, they are technically feasible only in few plant species that are amenable for infiltration and/or are hosts for the
viruses used as viral vectors. In this context, transgenic plant systems are more suited for wider adoption since broad range of plant species can be transformed using latest biotechnology
tools. When comparing our results of protein expression in sugarcane culms with other transgenic plant expression systems, caution was exercised particularly when comparing recovered protein
yields per starting tissue weight (e.g., mg/kg). This is because not all plant tissues have similar compositions, nor the protein extractions are equally efficient among tissue types, owing
to biological and biochemical differences41. For instance, sugarcane culms primarily constitute juice (sugars) and lignocellulosic fiber (bagasse). An equal amount of _N. benthamiana_
leaves on a fresh weight basis will have less fiber, and proteins may be easier to extract from leaf tissues. We also note that biochemical properties of target proteins such as size,
solubility, amino-acid composition, structural features, and protein stability may also ultimately influence the final yield. With these caveats in mind, we compared our results with other
reported studies of transgenic plant systems using the % TSP unit of recovered proteins. Several studies have reported recombinant protein yields of ~ 0.002 to 0.05% of TSP in transgenic
carrots47,48, ~ 0.23–2.5% of TSP in transgenic tobacco and potato49, ~ 8% TSP in transgenic tomato50, and ~ 11.9% in transgenic rice51. These comparisons suggest that higher protein yields
can be achieved using the sugarcane transgenic system (up to 11.5% of TSP), which are comparable to other transgenic systems, if not greater. In addition to the use of constitutive or
tissue-specific promoters, inducible promoters can be used for expressing recombinant proteins in plants52,53,54. Several inducible promoters can be used for generating transgenic plants
such as dexamethasone-, ethylene-, heat shock- and estradiol-inducible promoters52. Indeed, we have previously shown that the sugarcane DIRIGENT (SHDIR16) promoter is responsive to plant
hormones such as salicylic acid or jasmonic acid35. This is promising and suggests that inducible promoters such as SHDIR16, and other well-characterized plant inducible-promoters52 can be
further used in lieu or in combination with the constitutive/tissue-specific promoters that we have described, in order to robustly control and/or fine-tune the recombinant protein
expression. INCREASED PROTEIN LEVELS WERE ASSOCIATED WITH THE NUMBER OF COMBINATORIAL STACKED PROMOTERS AND NOT WITH THE COPY NUMBER ALONE Our results show that using multiple different
promoters to drive expression of recombinant _BvLz__m_ on distinct vectors enhanced recombinant protein accumulation. It is possible that the enhanced levels may have occurred due to higher
number of inserted _BvLz__m_ copies alone or it could be due to a combination of promoter-driven synergistic transcriptional activity. To test these scenarios, we performed a comparison of
the BvLzm transcript and yield among the various promoter stacked lines that had similar number of insertions. This analysis showed that there is a positive correlation in BvLzm transcript
and yield with combinatorial promoter:_BvLz__m_ stacks, irrespective of the number _BvLz__m_ inserts (Fig. 2; Supplementary Fig. S2). For instance, for single promoter:_BvLz__m_ line 13,
double promoter:_BvLz__m_ line 42, triple promoter:_BvLz__m_ line 20 and quadruple promoter:_BvLz__m_ line 10, with all of them having about 4–5 _BvLz__m_ inserts, there was a clear
enhancement in the BvLzm yield (Fig. 2a,c; Supplementary Fig. S2). Conversely, a comparison of single promoter:_BvLz__m_ transgenic lines with one or multiple inserts showed that there was
no corresponding increase in BvLz yield with the copy number. For instance, line 19 with one insert (Fig. 2a,c; Supplementary Fig. S2) had a BvLzm yield of 0.2 mg/kg, while line 13 with 4
_BvLz__m_ inserts had a BvLzm yield of 0.15 mg/kg (Fig. 2a, c; Supplementary Fig. S2). Together, these results suggest that the increase in BvLzm yield is primarily attributed to the number
of combinatorial stacked multiple promoters and not just with the _BvLz__m_ copy number alone. COMBINATORIAL PROMOTER STACKING MAY ALLEVIATE TRANSCRIPTIONAL OCCLUSION AND/OR RECOMBINANT GENE
SILENCING Multiple identical copies of recombinant genes or promoter transcription units (PTUs) delivered through a single construct could trigger transgene silencing55,56,57 or result in
promoter occlusion or transcriptional interference, a phenomenon observed in eukaryotic systems, including plants58,59,60,61,62. For instance, a strong PTU can sequester most of the
transcription factors in its immediate vicinity, limiting transcription from other promoters present in _cis_ on the same vector63. Alternatively, homology-dependent DNA methylation within
the promoter or in the coding region sequences could result in transgene silencing. For instance, in maize, transgenic lines with four copies of a cellulase gene, under control of tandemly
arranged PTUs on the same vector, resulted in lowered expression than those lines with fewer copies64. Our results here showed a positive correlation between the number of combinatorial
promoter stacks of recombinant _BvLz__m_ and increase in _BvLz__m_ levels, with no apparent transgene silencing. It is likely that using different promoter sequences in separate vectors may
overcome the transgene silencing or transcriptional interference. We suggest that each expression vector in the described stacked multiple promoter:_BvLz__m_ system (Fig. 1) does not
negatively affect the others, as shown by a positive correlation between the combinatorial promoter:_BvLz__m_ copy number (Table 2) and enhanced steady-state _BvLz__m_ transcript
accumulation (Fig. 2b; Supplementary Fig. S3) and BvLzm activity (Table 1; Fig. 2c). ELEVATED RECOMBINANT BVLZM ACCUMULATION POSITIVELY ENHANCES TRANSGENIC PLANT GROWTH Analysis of the
deleterious effects of recombinant protein accumulation on plant physiology and growth is crucial in order to assess the economic feasibility of using transgenic plants as biofactories, and
this is largely dependent on the target protein function65. In our scenario with BvLzm, we found no deleterious effects of enhanced _BvLz__m_ expression on sugarcane growth. On the contrary,
several growth characteristics of _BvLz__m_ expressing lines were better than those of non-transformed plants, such as enhanced leaf length, culm height, tiller number, culm biomass and
Brix (total soluble solids) (Table 3). These differences were statistically significant (_p_ < 0.001 and _p_ < 0.0001) in the triple and quadruple promoter:_BvLz__m_ expressing lines
(Table 3). For instance, mean culm fresh biomass per plant of the quadruple promoter:_BvLz__m_ expressing lines was nearly 2.5 times greater than that of non-transformed plants. The mean
soluble solids content in juice from triple and quadruple promoter:_BvLz__m_ expressing lines was approximately 20% higher than that of non-transformed plants. Similar trends were also
observed for leaf length, culm height and tiller density. The enhanced agronomic performance of the transgenic lines suggested that BvLzm, which is a well-known antimicrobial protein27,
could have a growth-promoting or perhaps protective role against pathogens present in the natural growth environment. RECOMBINANT PROTEIN ACCUMULATION IN CULMS INCREASES WITH PLANT AGE To
monitor the temporal stability of BvLzm accumulation in sugarcane culms in a growing season, we analyzed BvLzm levels for 11-months with a selection of several representative single promoter
pU:_BvLz_m lines. The BvLzm yields (mg of BvLzm/kg of harvested culm) in these lines after 7-, 9- and 11-month-harvest are shown in Fig. 4 (data for four representative lines) and Table 3
(data for six representative lines at the 11 month-harvest). There was a significant (_p_ < 0.05) increase in BvLzm yield over time for all the lines tested. BvLzm accumulation was
highest at the 11-month harvest, with lines 67, 108 and 114 showing the most significant (_p_ < 0.05) increase (Fig. 4). This accumulation pattern coincides with timing of culm ripening,
which is characterized by increased sucrose translocation and accumulation in culms. The age-related sucrose accumulation also was associated with the reduction in vegetative development
(leaf initiation and expansion) and commences at the mature basal internodes, progressing towards the culm apex, until the entire culm reaches a stable sugar level as it approaches
physiological maturity66. The age-related pattern of BvLzm accumulation may also be regulated by similar factors whereby photoassimilates and other substrates for BvLzm are diverted from
vegetative growth towards metabolite synthesis and accumulation during sugarcane maturation. Regardless of the mechanisms regulating the temporal accumulation of BvLzm, our results
demonstrate that the recombinant protein levels can be maintained, if not enhanced, during the development phases of sugarcane in a growing season. Similar results were observed for BvLzm
accumulation in representative triple promoter:_BvLz_m lines, which showed sustained and stable BvLzm levels over a full growing year, as well as in successive vegetative propagations
(Supplementary Table S3). Similar accumulation of recombinant proteins (human therapeutic interleukin-10) with plant maturity was observed in tobacco67. HIGH LEVEL RECOMBINANT PROTEIN
ACCUMULATION REQUIRES ADEQUATE MINERAL NUTRITION TO SUSTAIN THE PROTEIN AND BIOMASS ACCUMULATION Adequate water and nutrients supply are important for crop productivity as well as quality
considerations, such as protein content and other sensory traits68. Because we observed enhanced growth traits such as biomass in the _BvLz__m_ expressing lines, specifically in the triple
promoter:_BvLz__m_ lines (Table 3), we next investigated the optimal fertilization regime needed to sustain the additional growth and high levels of BvLzm production. Four representative
triple promoter:_BvLz__m_ lines (2-month old) were subjected to two mineral nutrient supply regimes namely, low fertility (LF or 2.4 mg N per plant, twice a week) and a high fertility (HF or
8 mg N per plant), using a balanced commercial fertilizer (Peters Professional 20–20–20; see “Materials and methods” section). BvLzm yield and growth traits were measured at 2-, 6-, and
8-months following fertilization. Supplemental fertilization increased culm biomass and BvLzm yield in the triple promoter:_BvLz__m_ lines over time. The most significant increases (_p_ <
0.05) between LF and HF were noted at 2 months for all lines (Fig. 5). For instance, pUPE:_BvLz__m_ line 32C (CP72-1210 variety) and pUDE:_BvLz_m lines 19, 44 and 54 (TCP98-4454 variety)
showed 4.6-, 2.5-, 3.0- and 2.0-fold increases in culm biomass and 1.7-, 1.3-, 1.1 and 1.0-fold enhancements in BvLzm yield, respectively. Leaf macronutrient contents of the triple
promoter:_BvLz__m_ plants were also monitored following growth under the two fertilization regimes. Plants grown under high nutrient supply rates had significantly (_p_ < 0.0001) higher
leaf mineral nutrient contents compared to those grown under low nutrient supply rates (Table 4). Leaves of HF plants had higher levels of N, phosphorus (P), potassium (K) and magnesium
(Mg), compared to leaves of LF plants (Table 4). In general, leaf nutrient content of the _BvLz__m_ expressing lines was improved by supplemental fertilization, resulting in a 1.5- to
2.2-fold increase in culm biomass and a subsequent 1.2- to 2.2-fold enhancement in BvLzm yield at 8 month-growth stage (Fig. 5). Taken together, the accumulation of _BvLz__m_ in response to
fertilization and the ontogenic _BvLz__m_ accumulation pattern underscore the need for adequate input availability to sustain not only biomass production but also the yield of high-value
proteins in crops such as sugarcane. CONCLUSIONS The genetic/biotechnology tools and resources developed in this study not only expands the utility of sugarcane for large-scale production of
recombinant proteins but can be utilized with other monocots and bioenergy feedstocks. Our approach comprises stacking multiple promoters to co-express codon-optimized recombinant genes
from different expression vectors using combinatorial transformation methods. This resulted in high recombinant protein yield (up to 11.5% of TSP or 82.5 mg/kg) in transgenic culms,
rendering it an attractive biopharming tool for potential commercial uses69. We also showed that recombinant BvLzm levels can be maintained stably throughout the growing season and had no
negative consequences on sugarcane agronomic performance. Overall, our study provides new knowledge, tools and resources to expand the utility of sugarcane beyond a food crop and bioenergy
feedstock to using it as a biofactory for expressing high-value proteins25. MATERIALS AND METHODS EXPRESSION VECTORS BASIC VECTORS A series of expression vectors were constructed, using a
custom synthesized bovine lysozyme (_BvLz_) gene codon-optimized for expression in maize (_BvLz__m_) (444.0 base pairs [bp])39 (GenScript, Piscataway, NJ). The _BvLz__m_ gene was subcloned
into pUC57 at _Bam_HI and cloned at the same site into pZero2 (Invitrogen, ThermoFisher Scientific, Waltham, MA), to which the 35ST34,38,39 (197.0 bp) was added at the _Pst_I site, resulting
in the _BvLz__m_-35ST/pZero2 plasmid. Three basic _BvLz_ expression vectors were generated with the constitutive promoters pUbi33, pSHPRP36 or pSHEF1α36. The first vector,
pUbi-_BvLz__m_-35ST/pZero2 was produced by cloning the pUbi fragment (1,977 bp), released from pAHC20 (pUbi:_BAR_/pUC8)70 (pUbi minus heat shock element; a 28.0 bp deletion at the 5′ end of
pUbi) with _Bam_HI/_Hind_III and filled in, into the filled-in _BvLz__m_-35ST/pZero2. For the other two vectors, the _Sma_I-treated pSHPRP (3,016 bp) and pSHEF1α (1,959 bp) fragments from
pSK+36 were fused to the _Sna_BI/_Bbs_I-treated/filled-in _BvLz__m_-35ST fragment from pUbi-_BvLz__m_-35ST/pZero2 to yield pSHPRP-_BvLz__m_-35ST/pSK+ and pSHEF1α-_BvLz__m_-35ST/pSK+,
respectively. Two basic _BvLz_ expression vectors were generated with the culm-regulated promoters pSHDIR1635 or pSCBV2134. The pSHDIR16-_BvLz__m_-35ST/pSK+ vector was assembled by fusing
_BvLz__m_-35ST, excised from _Bam_HI/_Eco_RI-treated _BvLz__m_-35ST/pZero2, to the pSHDIR16 fragment35 (2,680 bp) at the same sites in pSK+. The pSCBV21-_BvLz__m_-35ST/pGEMT-T Easy vector
was produced by cloning _BvLz__m_-35ST, excised from _Bam_HI/_Eco_RI-treated _BvLz__m_-35ST/pZero2, into the _Nco_I-treated/filled-in pSCVB21 (1,816 bp)/pGEM-T Easy34. DOUBLE TERMINATOR
VECTORS _BvLz_ constructs with a double terminator were generated by fusing the NOST (253 bp)39 to the 35ST of basic _BvLz_ constructs. The pUbi-_BvLz__m_-35STNOST/pZero2 vector was
constructed by releasing the NOST from pBI221 (Accession Number AF502128) (Clontech Laboratories, Inc., Mountain View, CA) with _Eco_RI/_Sst_I, filled in and cloned into the
_Xho_I-treated/filled-in pUbi-_BvLz__m_-35ST/pZero2. To make pSHPRP-_BvLz__m_-35STNOST/pSK+ and pSHEF1α-_BvLz__m_-35STNOST/pSK, the _Sna_BI/_Bbs_I-treated/filled-in _BvLz__m_-35STNOST
fragment from pUbi-_BvLz__m_-35STNOST/pZero2 was fused to the _Sma_I-treated pSHPRP/pSK+ and pSHEF1α/pSK+ vectors, respectively. To generate the pSCBV21-_BvLz__m_-35STNOST/pGEM-T Easy
vector, the _Sna_BI/_Bbs_I-treated/filled-in _BvLz__m_-35STNOST fragment from pUbi-_BvLz__m_-35STNOST/pZero2 was cloned into _Nco_I-treated/filled-in pSCVB21/pGEM-T Easy. VECTORS WITH VIRAL
UNTRANSLATED REGIONS The 3′UTR of SrMV strain H (GenBank Accession Number U57358) (235.0 bp) was custom synthesized as a fusion to _BvLz__m_ in pJI (_BvLz__m_-SrMV 3′UTR/pJI) (ATUM, DNA2.0,
Newark, CA). The pUbi-_BvLz__m_-SrMV 3′UTR-35ST/pZero2 vector was assembled by cloning the filled-in SrMV 3′UTR, released from _Eco_RI/_Bgl_II-treated _BvLz__m_-3′SrMV/pJI, into
pUbi-_BvLz__m_-35ST/pZero2 at the _Sma_I site. The pSHPRP-_BvLz__m_-SrMV 3′UTR-35ST/pSK+ and pSHEF1α-_BvLz__m_-SrMV 3′UTR-35ST/pSK+ vectors were generated by fusing the
_Sna_BI/_Bbs_I-treated/filled-in _BvLz__m_-SrMV 3′UTR-35ST fragment from the pUbi-_BvLz__m_-SrMV 3′UTR-35ST/pZero2 to pSHPRP/pSK+ and pSHEF1α/pSK+ at the _Sma_I site, respectively. For
construction of pSHDIR16_-BvLz__m_-SrMV 3′UTR-35ST/pSK+ vector, SrMV 3′UTR was released from _BvLz__m_-SrMV 3′UTR/pJI by _Eco_RV treatment and cloned into pSHDIR16-_BvLz__m_-35ST/pSK+ at the
_Eco_RV site. All DNA cloning steps were carried out as described by Sambrook71. Filling in of endonuclease-treated DNA fragments and dephosphorylation of vectors were done using T4 DNA
polymerase (NEB BioLabs, Ipswich, MA) and antarctic phosphatase (NEB BioLabs), respectively. SUGARCANE TRANSFORMATION Tops of field-grown sugarcane (_Saccharum_ spp. hybrids) commercial
varieties CP72-1210, CP84-1198, TCP87-3388 and TCP98-4454 were collected during the growing season, and leaf roll discs were prepared for stable transformations as previously described72.
Briefly, leaf blades and sheaths were removed down to the top visible dewlap leaf, and the upper 20–30 cm portion of shoot (leaf roll culm) was surface sterilized in 70.0% (v/v) ethanol for
20 min. Immature leaf rolls close to the apical meristem were sliced transversely into 1.0 mm thick sections and cultured on MS3 medium (MS medium with 3.0 mg/l of 2,4-dichlorophenoxyacetic
acid [2,4-D]) for 30–35 days (for embryogenic calli) or MS0.6 medium (MS with 0.6 mg/l of 2,4-D) for 7–10 days (for embryogenic leaf roll discs). Embryogenic calli and leaf roll discs were
preconditioned on MS3- and MS0.6-osmoticum (MS3 or MS0.6 with 0.2 M d-mannitol and 0.2 M d-sorbitol), respectively, for 4 h before and after DNA particle bombardment. DNA bombardment was
performed according to Beyene and colleagues38. Briefly, tungsten particles (1.1 µm; Bio-Rad Laboratories, Inc.) (1.0 mg) were coated separately with plasmid DNA (1.0 µg) of different
constructs at equimolar ratios together with pUbi:_BAR_/pUC8 selectable marker plasmid using calcium chloride (NaCl) (1.0 M) and spermidine (14.0 mM). The DNA particle suspension (containing
the selectable marker plasmid with one or more _BvLz__m_ plasmids) (4.0 μl; 0.5 µg DNA per bombardment) was placed at the center of a syringe filter and delivered into tissue with a
particle inflow gun using a 26.0-inch Hg vacuum and a 7.0-cm target distance. Bombarded embryogenic calli and leaf roll discs were maintained on MS3 and MS0.6, respectively, for 10 days in
the dark at 28 °C for recovery. They were later incubated in the dark at 28 °C on selection medium (MS3 or MS0.6 with bialaphos at 3.0 mg/l) for a total of 2 weeks. Shoot regeneration and
root initiation were performed under bialaphos selection as previously described72. Rooted plantlets were transferred to potting soil (Sunshine Mix #1; SunGro Horticulture Distribution,
Inc., Agawan, MA) in pots and maintained in the greenhouse. TRANSGENIC PLANT SCREENING INTEGRATION AND SIZE DETERMINATION OF _BVLZ__M_ EXPRESSION CASSETTES Integration and size of each
_BvLz__m_ expression cassette in the single and multiple stacked promoter:_BvLz__m_ sugarcane lines were determined by Southern blot and PCR analyses, respectively, using genomic DNA
isolated according to Tai and Tanksley73 from liquid N-ground tissues (3.0 g) collected from young leaves of 3–4 month-old plants. Controls included vector-transformed lines and
non-transformed plants (tissue culture-derived). For Southern blot analysis, genomic DNA (10.0 μg per lane) was treated with _Hind_III endonuclease, electrophoresed on 0.8% (w/v) agarose
gels and transferred to nylon membranes (Amersham Hybond-XL, GE Healthcare Bio-Sciences Corp., Piscataway, NJ) in 0.4 M sodium hydroxide74. Pre-hybridization, hybridization, washing and
detection of DNA gel blots were performed using Church’s buffer75. The probe, corresponding to the _BvLz__m_ coding sequence was amplified by PCR from pUbi-_BvLz__m_-35ST/pZero2 using the
primer set BvLz-1F (5′-ATGGCGGCCCTGGTGATCCTGGGCT-3′) and BvLz-481R (5′-TCACAGGGTGCAGCCTTCCACG-3′) and labeled with [α-32P] dCTP using the Random DNA Labeling kit (Invitrogen, ThermoFisher
Scientific). PCR was performed on a C1000 Touch thermal cycler (Bio-Rad Laboratories, Inc., Hercules, CA) in a total reaction volume of 25.0 µl using 200.0 ng of DNA and Platinum _Taq_ DNA
polymerase (Invitrogen, ThermoFisher Scientific) according to the manufacturer’s instructions with the following conditions: 94 °C for 4 min, 35 cycles each at 94 °C for 30 s, 49.7–54.4 °C
for 30 s, and 72 °C for 6 min. Primers encompassing the entire promoter:_BvLz__m_-terminator cassette (Supplementary Table S4) were designed with Primer 3.0. All PCR amplicons were separated
by electrophoresis on 0.7% agarose (w/v) gels stained with ethidium bromide. A “no DNA template” was included as a negative control for PCR. DETERMINATION OF _BVLZ__M_ COPY NUMBER _BvLz__m_
copy number in single and multiple stacked promoter:_BvLz__m_ sugarcane lines was estimated by qPCR. qPCR was performed on a CFX384 Real-time PCR Detection System (Bio-Rad Laboratories,
Inc.) using i_Taq_ Universal SYBR Green Supermix (Bio-Rad Laboratories, Inc.), 0.4 µM of each target specific primer and 1.0 ng of genomic DNA from representative transgenic _BvLz__m_ lines,
according to the manufacturer’s instructions. Primers specific to the promoter-_BvLz__m_ gene junction area (Supplementary Table S4) were designed with Primer 3.0
(https://bioinfo.ut.ee/primer3-0.4.0/primer3/). qPCR conditions were as follows: 95.0 °C for 3 min, 39 two-step cycles each at 96.0 °C for 5 s and 57 °C for 30 s, and a final melting curve
of 60.0 °C to 95.0 °C for 6 min. The sugarcane anthranilate phosphoribosyltransferase and prolyl 4-hydroxylase genes were used as a reference for single copy genes76. qPCR was performed
twice in triplicate with two biological replications. PCR efficiency was calculated with LinReg77. Results were analyzed and recorded as CT (threshold cycle) values. Copy number of the
_BvLz__m_ gene was estimated by qPCR according to Casu et al.76 using the formula GCI = EffRefCT/EffCT, where: GCI = gene copy number index, EffRefCT = PCR efficiency using the reference
gene primers to the power of the reference gene CT value for each sample, and EffCT = PCR efficiency using the test gene primers to the power of the test gene CT value generated for each
sample. EXPRESSION ANALYSIS OF _BVLZ__M_ Total RNA was isolated by grinding 1.0 g of young leaves collected from 3–4 month-old plants in liquid N39,78. For northern blot analysis, RNA (15.0
μg per lane) was fractionated on 1.6% formaldehyde agarose denaturing gels in HEPES buffer and blotted onto nylon membranes (Amersham Hybond-XL) in 10x SSC75. Pre-hybridization, _BvLz__m_
probe labeling, hybridization, washing and detection of RNA gel blots were performed as described for Southern blot analysis. PLANT GROWTH AND TREATMENT CONDITIONS For growth cycle
investigations, single-node culm cuttings of 15 single promoter pU:_BvLz__m_ transgenic lines and non-transformed plants were pre-germinated in seedling flats (Supplementary Fig. S1) for 2.5
weeks and transplanted into 37.0-l pots (four pots per line) in commercial growth medium (Sunshine Mix #1). Plants were maintained in a temperature-regulated greenhouse with average
day/night temperatures of 32/22 °C and relative humidity of 60–100%. Plants were initially fertilized once per week with a commercial high-phosphorus soluble fertilizer (Peters
8%N-19.8%P-12.5%K; The Scotts Company, Marysville, OH) for 5 weeks and then with a balanced/complete soluble fertilizer (Peters Professional 20–20–20; The Scotts Company) containing N 200.0
g/kg, P 80.0 g/kg, K 166.0 g/kg, Mg 1.0 g/kg, iron 0.5 g/kg, manganese 0.3 g/kg, boron 0.1 g/kg, copper 0.13 g/kg, molybdenum 0.05 g/kg, and zinc 0.25 g/kg. To assess the impacts of mineral
nutrient supply on growth and BvLzm accumulation, plants from four representative triple promoter pUDE:_BvLz__m_ lines and one representative triple promoter pUPE:_BvLz__m_ line were
pre-germinated and transplanted into 15.0-l plastic pots containing the same growth medium as described above. All pots were initially fertilized with a high-phosphorus fertilizer (Peters
8%N–19.8%P–12.5%K; Scotts, Marysville, OH; equivalent to 10.0 kg N/ha). After 2 months, pots were randomly assigned into two fertilization treatment groups, namely, high fertility (HF) and
low fertility (LF), with four pots per line selected for each group. Non-transformed plants (tissue culture-derived) were included as negative controls. Fertilization treatments were
achieved with a complete fertilizer (Peters Professional 20–20–20) containing macro- and micro-nutrients as described above. Plants in the LF group received an additional equivalent of 20.0
kg N/ha whereas HF plants received 50.0 kg N/ha from supplemental fertilization using Peters Professional 20–20–20 (described above). Fertilizer treatments were applied in split doses (twice
per week). Transgenic culms were harvested at 2, 6 and 8 months following fertilization, processed, and their BvLz yield was determined by ELISA at the BioSeparation Facility of Texas
A&M University’s Biological and Agricultural Engineering Department (College Station, Texas). PLANT PHYSIOLOGICAL ANALYSIS For inorganic mineral analysis, leaf tissue samples were
collected, dried (70 °C for 48 h), ground to pass a 40-μm screen and analyzed for inorganic minerals. Total Kjeldahl N (ammonia and organic N) was determined in digested samples using the
EasyChem Plus Analyzer and protocols (Systea Scientific, Chicago, IL), whereas other macronutrients such as P, K and Mg were analyzed using the Optima 7300 DV Inductively Coupled
Plasma-Optical Emission Spectrometer (PerkinElmer, Shelton, CT) after partial digestion (hydrolysis) on a HotBlock Digestion System (Environmental Express, Inc., Charleston, SC). TOTAL
PROTEIN EXTRACTION Large-scale extraction and size fractionation of total soluble proteins (TSPs) from culms (300.0 lbs) of _BvLz__m_ transgenic sugarcane were performed at our Pilot Plant
Facility mainly as described previously79. Bench-scale extraction and purification of BvLzm from extracts of transgenic sugarcane culms (100.0 g), using a single-step hydrophobic interaction
chromatography, were performed at our BioSeparation Facility (College Station, Texas) as previously described30. For small-scale extraction of TSP from _BvLz__m_ transgenic sugarcane leaf
tissue (200.0 mg) was homogenized in 600.0 µl of sodium acetate buffer (50 mM NaOAc, pH 4.4, 0.1 M NaCl) in 2.0 ml tubes for 30 s at 5,000 rpm with the Precellys 24 homogenizer (MO BIO
Laboratories, Carlsbad, CA) using ceramic spherical beads (0.64 cm-diameter). TSP supernatants were collected by centrifugation at 13,000_g_ for 25 min at 4 °C. DETERMINATION OF BVLZM
ACCUMULATION BY ENZYME ACTIVITY AND ENZYME-LINKED IMMUNOSORBENT ASSAYS To determine the levels of recombinant BvLzm, enzyme activity and enzyme-linked immunosorbent assays (ELISA) were
performed on TSP from culm extract juice. Juice was extracted from 1.0 kg of culms of greenhouse grown _BvLz__m_ transgenic plants at 7, 9 and 11 months for the growth cycle experiment and
at 2, 6 and 8 months for the fertilization experiment. For enzyme activity determination, culm extract juice was tested for its ability to lyse _Micrococcus lysodeikticus_ cells using the
standard protocol from Sigma-Aldrich (St. Louis, MO). Rabbit anti-BvLz antibody used in the ELISA was synthesized by Bethyl Laboratories, Inc. (Montgomery, TX) using tobacco-derived BvLz31
and further purified through an SP-Sepharose column (GE Healthcare, Piscataway, NJ). ELISA of culm extract juice was performed as previously described30. Briefly, a sandwich ELISA consisting
of anti-BvLz antibody was used to capture BvLz in juice. Detection was performed using a biotinylated anti-BvLz antibody and horseradish peroxidase-labeled NeutrAvidin (Pierce, ThermoFisher
Scientific). The standard curve was generated using BvLz produced in _Pichia pastoris_ as in Digan et al.80. STATISTICAL ANALYSIS Agronomic data were collected from 3 to 4 independent
experiments, with 3–4 replicates per experiment and subjected to an analysis of variance (ANOVA) using the General Linear Model procedure of the Statistical Analysis System 9.4 (SAS
Institute Inc., Cary, NC). Mean separation was performed using the Student–Newman–Keuls (SNK) test. REFERENCES * Demain, A. & Vaishnav, P. Production of recombinant proteins by microbes
and higher organisms. _Biotechnol. Adv._27, 297–306. https://doi.org/10.1016/j.biotechadv.2009.01.008 (2009). Article PubMed CAS Google Scholar * Basaran, P. & Rodríguez-Cerezo, E.
Plant molecular farming: opportunities and challenges. _Crit. Rev. Biotechnol._28, 153–172. https://doi.org/10.1080/07388550802046624 (2008). Article PubMed Google Scholar * Rybicki, E.
P. Plant-made vaccines for humans and animals. _Plant Biotechnol. J._8, 620–637 (2010). PubMed PubMed Central CAS Google Scholar * Wiktorek-Smagur, A. _et al._ Green way of
biomedicine—how to force plants to produce new important proteins. In _Transgenic Plants-Advances and Limitations_ (ed. Çiftçi, Y. O.) 63–90 (InTech, Rijeka, 2012). Google Scholar * Twyman,
R. M., Stoger, E., Schillberg, S., Christou, P. & Fischer, R. Molecular farming in plants: host systems and expression technology. _Trends Biotechnol._21, 570–578 (2003). PubMed CAS
Google Scholar * Brumbley, S. M., Purnell, M. P., Petrasovits, L. A., Nielsen, L. K. & Twine, P. H. Developing the sugarcane biofactory for high-value biomaterials. _Int. Sugar J._1297,
5 (2007). Google Scholar * Ando, S. _et al._ Overwintering ability and dry matter production of sugarcane hybrids and relatives in the Kanto Region of Japan. _Jpn. Agric. Res. Q._45,
259–267. https://doi.org/10.6090/jarq.45.259 (2011). Article Google Scholar * Matsuoka, S., Kennedy, A. J., Santos, E. G. D., Tomazela, A. L. & Rubio, L. C. S. Energy cane: its
concept, development, characteristics, and prospects. _Adv. Bot._2014, 597275 (2014). Google Scholar * Gallo-Meagher, M. & Irvine, J. E. Effects of tissue type and promoter strength on
transient GUS expression in sugarcane following particle bombardment. _Plant Cell Rep._12, 666–670. https://doi.org/10.1007/BF00233416 (1993). Article PubMed CAS Google Scholar *
Lakshmanan, P. _et al._ Sugarcane biotechnology: the challenges and opportunities. _Vitro Cell. Dev. Biol. Plant_41, 345–363 (2005). CAS Google Scholar * Anderson, D. J. _et al._ Synthesis
of short-chain-length/medium-chain length polyhydroxyalkanoate (PHA) copolymers in peroxisomes of transgenic sugarcane plants. _Trop. Plant Biol._4, 170–184.
https://doi.org/10.1007/s12042-011-9080-7 (2011). Article CAS Google Scholar * McQualter, R. B. _et al._ Initial evaluation of sugarcane as a production platform for p-hydroxybenzoic
acid. _Plant Biotechnol. J._3, 29–41 (2005). PubMed CAS Google Scholar * Petrasovits, L. A. _et al._ Enhanced polyhydroxybutyrate production in transgenic sugarcane. _Plant Biotechnol.
J._10, 569–578 (2012). PubMed CAS Google Scholar * Purnell, M. P., Petrasovits, L. A., Nielsen, L. K. & Brumbley, S. M. Spatio-temporal characterization of polyhydroxybutyrate
accumulation in sugarcane. _Plant Biotechnol. J._5, 173–184 (2007). PubMed CAS Google Scholar * Tilbrook, K., Gebbie, L., Schenk, P. M., Poirier, Y. & Brumbley, S. M. Peroxisomal
polyhydroxyalkanoate biosynthesis is a promising strategy for bioplastic production in high biomass crops. _Plant Biotechnol. J._9, 958–969 (2011). PubMed CAS Google Scholar * Chong, B.
F. _et al._ Co-ordinated synthesis of gentiobiitol and sorbitol, evidence of sorbitol glycosylation in transgenic sugarcane. _Phytochemistry_71, 736–741.
https://doi.org/10.1016/j.phytochem.2010.01.014 (2010). Article PubMed CAS Google Scholar * Mudge, S. R. _et al._ Mature-stem expression of a silencing-resistant sucrose isomerase gene
drives isomaltulose accumulation to high levels in sugarcane. _Plant Biotechnol. J._11, 502–509 (2013). PubMed CAS Google Scholar * Wu, L. & Birch, R. G. Doubled sugar content in
sugarcane plants modified to produce a sucrose isomer. _Plant Biotechnol. J._5, 109–117. https://doi.org/10.1111/j.1467-7652.2006.00224.x (2007). Article PubMed CAS Google Scholar *
Wang, M.-L., Goldstein, C., Su, W., Moore, P. H. & Albert, H. H. Production of biologically active GM-CSF in sugarcane: a secure biofactory. _Transgenic Res._14, 167–178 (2005). PubMed
Google Scholar * Henrique-Silva, F. & Soares-Costa, A. _Transgenic Plants_ 437–450 (Springer, Berlin, 2012). Google Scholar * Gianotti, A. _et al._ Recombinant expression,
purification, and functional analysis of two novel cystatins from sugarcane (_Saccharum officinarum_). _Protein Expr. Purif._47, 483–489. https://doi.org/10.1016/j.pep.2005.10.026 (2006).
Article PubMed CAS Google Scholar * Ribeiro, C. W. _et al._ Production of a His-tagged canecystatin in transgenic sugarcane and subsequent purification. _Biotechnol. Prog._24, 1060–1066
(2008). PubMed CAS Google Scholar * Harrison, M. D. _et al._ Accumulation of recombinant cellobiohydrolase and endoglucanase in the leaves of mature transgenic sugar cane. _Plant
Biotechnol. J._9, 884–896. https://doi.org/10.1111/j.1467-7652.2011.00597.x (2011). Article PubMed CAS Google Scholar * Harrison, M. D. _et al._ Recombinant cellulase accumulation in the
leaves of mature, vegetatively propagated transgenic sugarcane. _Mol. Biotechnol._56, 795–802 (2014). PubMed CAS Google Scholar * Palaniswamy, H. _et al._ Vacuolar targeting of
r-proteins in sugarcane leads to higher levels of purifiable commercially equivalent recombinant proteins in cane juice. _Plant Biotechnol. J._14, 791–807 (2016). PubMed CAS Google Scholar
* Jolles, P. _et al._ Stomach lysozymes of ruminants. II. Amino acid sequence of cow lysozyme 2 and immunological comparisons with other lysozymes. _J. Biol. Chem._259, 11617–11625 (1984).
PubMed CAS Google Scholar * Sahoo, N. R. _et al._ Lysozyme in livestock: a guide to selection for disease resistance: a review. _J. Anim. Sci. Adv._2, 347–360 (2012). Google Scholar *
Lemos, F. J. A., Ribeiro, A. F. & Terra, W. R. A bacteria-digesting midgut-lysozyme from _Musca domestica_ (Diptera) larvae. Purification, properties and secretory mechanism. _Insect
Biochem. Mol. Biol._23, 533–541 (1993). CAS Google Scholar * Mirkov, T. E. & Fitzmaurice, L. C. Google patents (1995). * Barros, G. O. F. _et al._ Recovery of bovine lysozyme from
transgenic sugarcane stalks: extraction, membrane filtration, and purification. _Bioprocess Biosyst. Eng._36, 1407–1416. https://doi.org/10.1007/s00449-012-0878-y (2013). Article PubMed
CAS Google Scholar * Wilcox, C. P. _et al._ Production and purification of an active bovine lysozyme in tobacco (_Nicotiana tabacum_): utilization of value-added crop plants traditionally
grown under intensive agriculture. _J. Agric. Food Chem._45, 2793–2797 (1997). CAS Google Scholar * Bock, R. Strategies for metabolic pathway engineering with multiple transgenes. _Plant
Mol. Biol._83, 21–31. https://doi.org/10.1007/s11103-013-0045-0 (2013). Article PubMed CAS Google Scholar * Christensen, A. H., Sharrock, R. A. & Quail, P. H. Maize polyubiquitin
genes: structure, thermal perturbation of expression and transcript splicing, and promoter activity following transfer to protoplasts by electroporation. _Plant Mol. Biol._18, 675–689.
https://doi.org/10.1007/BF00020010 (1992). Article PubMed CAS Google Scholar * Gao, S.-J. _et al._ A novel Sugarcane bacilliform virus promoter confers gene expression preferentially in
the vascular bundle and storage parenchyma of the sugarcane culm. _Biotechnol. Biofuels_10, 172–172. https://doi.org/10.1186/s13068-017-0850-9 (2017). Article PubMed PubMed Central CAS
Google Scholar * Damaj, M. B. _et al._ Sugarcane DIRIGENT and O-METHYLTRANSFERASE promoters confer stem-regulated gene expression in diverse monocots. _Planta_231, 1439–1458.
https://doi.org/10.1007/s00425-010-1138-5 (2010). Article PubMed CAS Google Scholar * Yang, M., Bower, R., Burow, M. D., Paterson, A. H. & Mirkov, T. E. A rapid and direct approach
to identify promoters that confer high levels of gene expression in monocots. _Crop Sci._43, 1805–1813 (2003). CAS Google Scholar * Woodard, S. L. _et al._ American Society of Agricultural
and Biological Engineers. * Beyene, G. _et al._ Unprecedented enhancement of transient gene expression from minimal cassettes using a double terminator. _Plant Cell Rep._30, 13–25.
https://doi.org/10.1007/s00299-010-0936-3 (2011). Article PubMed CAS Google Scholar * Damaj, M. B. & Mirkov, T. E. Compositions, organisms, systems, and methods for expressing a gene
product in plants. U.S. patent (2019). * Moon, K.-B. _et al._ Development of systems for the production of plant-derived biopharmaceuticals. _Plants_9, 30 (2020). CAS Google Scholar *
Merlin, M., Gecchele, E., Capaldi, S., Pezzotti, M. & Avesani, L. Comparative evaluation of recombinant protein production in different biofactories: the green perspective. _BioMed Res.
Int._2014, 1–14 (2014). Google Scholar * Jiang, M.-C., Hu, C.-C., Lin, N.-S. & Hsu, Y.-H. Production of human IFNγ protein in _Nicotiana benthamiana_ plant through an enhanced
expression system based on bamboo mosaic virus. _Viruses_11, 509 (2019). PubMed Central CAS Google Scholar * Zischewski, J., Sack, M. & Fischer, R. Overcoming low yields of plant-made
antibodies by a protein engineering approach. _Biotechnol. J._11, 107–116 (2016). PubMed CAS Google Scholar * Buyel, J. F., Twyman, R. M. & Fischer, R. Very-large-scale production of
antibodies in plants: the biologization of manufacturing. _Biotechnol. Adv._35, 458–465 (2017). PubMed CAS Google Scholar * Sainsbury, F. _et al._ Rapid transient production in plants by
replicating and non-replicating vectors yields high quality functional anti-HIV antibody. _PLoS ONE_5, e13976 (2010). ADS PubMed PubMed Central Google Scholar * Lai, H., He, J., Engle,
M., Diamond, M. S. & Chen, Q. Robust production of virus-like particles and monoclonal antibodies with geminiviral replicon vectors in lettuce. _Plant Biotechnol. J._10, 95–104 (2012).
PubMed CAS Google Scholar * Porceddu, A. _et al._ Transgenic plants expressing human glutamic acid decarboxylase (GAD65), a major autoantigen in insulin-dependent diabetes mellitus. _Mol.
Breed._5, 553–560 (1999). CAS Google Scholar * Uvarova, E. A. _et al._ Oral immunogenicity of plant-made _Mycobacterium tuberculosis_ ESAT6 and CFP10. _BioMed Res. Int._2013, 1–13 (2013).
Google Scholar * Mason, H. S. _et al._ Expression of Norwalk virus capsid protein in transgenic tobacco and potato and its oral immunogenicity in mice. _Proc. Natl. Acad. Sci._93,
5335–5340 (1996). ADS PubMed CAS Google Scholar * Zhang, X., Buehner, N. A., Hutson, A. M., Estes, M. K. & Mason, H. S. Tomato is a highly effective vehicle for expression and oral
immunization with Norwalk virus capsid protein. _Plant Biotechnol. J._4, 419–432 (2006). PubMed CAS Google Scholar * Tokuhara, D. _et al._ Rice-based oral antibody fragment prophylaxis
and therapy against rotavirus infection. _J. Clin. Investig._123, 3829–3838 (2013). PubMed CAS Google Scholar * Borghi, L. _Plant Developmental Biology_ 65–75 (Springer, Berlin, 2010).
Google Scholar * Maruyama, K. _et al._ Design of an optimal promoter involved in the heat-induced transcriptional pathway in Arabidopsis, soybean, rice and maize. _Plant J._89, 671–680
(2017). PubMed CAS Google Scholar * Gatz, C. Chemically inducible promoters in transgenic plants. _Curr. Opin. Biotechnol._7, 168–172 (1996). CAS Google Scholar * Butaye, K. M. J.,
Cammue, B. P. A., Delauré, S. L. & De Bolle, M. F. C. Approaches to minimize variation of transgene expression in plants. _Mol. Breed._16, 79–91.
https://doi.org/10.1007/s11032-005-4929-9 (2005). Article Google Scholar * Kohli, A. _et al._ The quest to understand the basis and mechanisms that control expression of introduced
transgenes in crop plants. _Plant Signal. Behav._1, 185–195. https://doi.org/10.4161/psb.1.4.3195 (2006). Article PubMed PubMed Central Google Scholar * Rajeev Kumar, S., Anunanthini, P.
& Ramalingam, S. Epigenetic silencing in transgenic plants. _Front. Plant Sci._6, 693. https://doi.org/10.3389/fpls.2015.00693 (2015). Article Google Scholar * Greger, I. H.,
Proudfoot, N. J., Demarchi, F. & Giacca, M. Transcriptional interference perturbs the binding of Sp1 to the HIV-1 promoter. _Nucleic Acids Res._26, 1294–1300.
https://doi.org/10.1093/nar/26.5.1294 (1998). Article PubMed PubMed Central CAS Google Scholar * Ingelbrecht, I. _et al._ Transcriptional interference in transgenic plants. _Gene_109,
239–242 (1991). PubMed CAS Google Scholar * Kadesch, T. & Berg, P. Effects of the position of the simian virus 40 enhancer on expression of multiple transcription units in a single
plasmid. _Mol. Cell. Biol._6, 2593–2601 (1986). PubMed PubMed Central CAS Google Scholar * Maqbool, S. B. & Christou, P. Multiple traits of agronomic importance in transgenic indica
rice plants: analysis of transgene integration patterns, expression levels and stability. _Mol. Breed._5, 471–480 (1999). Google Scholar * Shearwin, K. E., Callen, B. P. & Egan, J. B.
Transcriptional interference—a crash course. _Trends Genet._21, 339–345 (2005). PubMed PubMed Central CAS Google Scholar * Horlick, R. A. _et al._ Combinatorial gene expression using
multiple episomal vectors. _Gene_243, 187–194 (2000). PubMed CAS Google Scholar * Egelkrout, E. _et al._ Enhanced expression levels of cellulase enzymes using multiple transcription
units. _BioEnergy Res._6, 699–710 (2013). CAS Google Scholar * Urreta, I. & Castañón, S. Transgenic plants as biofactories for the production of biopharmaceuticals: A case study of
human placental lactogen. In _Transgenic Plants-Advances and Limitations_ (ed. Çiftçi, Y. O.) 305–328 (InTech, Rijeka, 2012). Google Scholar * Legendre, B. L. Ripening of sugarcane: effects
of sunlight, temperature, and rainfall 1. _Crop Sci._15, 349–352 (1975). Google Scholar * Duwadi, K., Chen, L., Menassa, R. & Dhaubhadel, S. Identification, characterization and
down-regulation of cysteine protease genes in tobacco for use in recombinant protein production. _PLoS ONE_10, e0130556 (2015). PubMed PubMed Central Google Scholar * Marschner, H.
_Mineral Nutrition of Higher Plants_ (Academic Press, NY, 1995). Google Scholar * Davies, H. M. Review article: commercialization of whole-plant systems for biomanufacturing of protein
products: evolution and prospects. _Plant Biotechnol. J._8, 845–861. https://doi.org/10.1111/j.1467-7652.2010.00550.x (2010). Article PubMed Google Scholar * Christensen, A. H. &
Quail, P. H. Ubiquitin promoter-based vectors for high-level expression of selectable and/or screenable marker genes in monocotyledonous plants. _Transgenic Res._5, 213–218.
https://doi.org/10.1007/BF01969712 (1996). Article PubMed CAS Google Scholar * Sambrook, H. C. _Molecular Cloning: A Laboratory Manual_ (Cold Spring Harbor Laboratory Press, Cold Spring
Harbor, 1989). Google Scholar * Ramasamy, M. _et al._ A biolistic-based genetic transformation system applicable to a broad-range of sugarcane and energycane varieties. _GM Crops Food_9,
211–227 (2018). PubMed PubMed Central Google Scholar * Tai, T. H. & Tanksley, S. D. A rapid and inexpensive method for isolation of total DNA from dehydrated plant tissue. _Plant Mol.
Biol. Report._8, 297–303 (1990). Google Scholar * Sambrook, J. & Russell, D. W. _Molecular Cloning: A Laboratory Manual_ (Cold Spring Harbor Laboratory Press, Cold Spring Harbor,
2001). Google Scholar * Mangwende, T. _et al._ The P0 gene of Sugarcane yellow leaf virus encodes an RNA silencing suppressor with unique activities. _Virology_384, 38–50 (2009). PubMed
CAS Google Scholar * Casu, R. E., Selivanova, A. & Perroux, J. M. High-throughput assessment of transgene copy number in sugarcane using real-time quantitative PCR. _Plant Cell
Rep._31, 167–177. https://doi.org/10.1007/s00299-011-1150-7 (2012). Article PubMed CAS Google Scholar * Ruijter, J. M. _et al._ Amplification efficiency: linking baseline and bias in the
analysis of quantitative PCR data. _Nucleic Acids Res._37, e45–e45 (2009). PubMed PubMed Central CAS Google Scholar * Damaj, M. B. _et al._ Reproducible RNA preparation from sugarcane
and citrus for functional genomic applications. _Int. J. Plant Genom._2009, 13. https://doi.org/10.1155/2009/765367 (2009). Article CAS Google Scholar * Mirkov, T. E., Monclin, J. P.,
Barrilleaux, A., Irvine, J. E. & Moonan, F. Google patents (2002). * Digan, M. E. _et al._ Continuous production of a novel lysozyme via secretion from the yeast, _Pichia pastoris_.
_Biotechnology_7, 160 (1989). CAS Google Scholar Download references ACKNOWLEDGEMENTS We would like to dedicate this manuscript to late Professor T. Erik Mirkov (1959–2018), a pioneer in
sugarcane biotechnology. We gratefully acknowledge Xavier Gonzales for the assembly of three genetic constructs, Renesh Bedre for the statistical analysis of data and Sonia Irigoyen (Texas
A&M AgriLife Research) for critical review of the manuscript. We are grateful to Denise Rossi, Hyun Park Kang, Ninfa Ramos, Soledad Al-Varez, Gerleene Acuna, Adan Solis and Abigail Cruz
for excellent technical assistance. This research was supported by funds from BioCane, Inc., a subsidiary of Grower Research Group LLC (Soledad, CA) and Texas A&M AgriLife Research
Grants (124738-96210; 124190-96210) to K.K.M. AUTHOR INFORMATION Author notes * Carol Vargas-Bautista Present address: College of Medicine, Texas A&M University, 8447 Riverside Parkway,
Bryan, TX, 77807, USA AUTHORS AND AFFILIATIONS * Texas A&M AgriLife Research and Extension Center, 2415 East US Highway 83, Weslaco, TX, 78596, USA Mona B. Damaj, John L. Jifon, Carol
Vargas-Bautista, Joe Molina & Kranthi K. Mandadi * Department of Horticultural Sciences, Texas A&M University, College Station, TX, 77843-2133, USA John L. Jifon * National Center
for Therapeutics Manufacturing, Texas A&M University, 100 Discovery Drive, College Station, TX, 77843-4482, USA Susan L. Woodard * BioSeparation Laboratory, Biological and Agricultural
Engineering Department, College Station, TX, 77843-2117, USA Georgia O. F. Barros, Steven G. White & Zivko L. Nikolov * Innovus Pharmaceuticals, Inc., 8845 Rehco Road, San Diego, CA,
92121, USA Bassam B. Damaj * Department of Plant Pathology and Microbiology, Texas A&M University, College Station, TX, 77843-2132, USA Kranthi K. Mandadi Authors * Mona B. Damaj View
author publications You can also search for this author inPubMed Google Scholar * John L. Jifon View author publications You can also search for this author inPubMed Google Scholar * Susan
L. Woodard View author publications You can also search for this author inPubMed Google Scholar * Carol Vargas-Bautista View author publications You can also search for this author inPubMed
Google Scholar * Georgia O. F. Barros View author publications You can also search for this author inPubMed Google Scholar * Joe Molina View author publications You can also search for this
author inPubMed Google Scholar * Steven G. White View author publications You can also search for this author inPubMed Google Scholar * Bassam B. Damaj View author publications You can also
search for this author inPubMed Google Scholar * Zivko L. Nikolov View author publications You can also search for this author inPubMed Google Scholar * Kranthi K. Mandadi View author
publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS M.B.D., J.L.J., S.L.W., C.V.-B., G.O.F.B., J.M., Z.L.N., and K.K.M. designed the experiments. B.B.D.
designed the combinatorial gene expression system and developed total protein enrichment protocols. M.B.D. made the genetic constructs and prepared the manuscript. M.B.D. and J.M. conducted
transformation experiments. M.B.D., J.M. and C.V.-B. conducted transgenic plant screening analyses. J.L.J. conducted growth cycle experiments. M.B.D. and J.M. conducted fertilization
experiments. S.L.W., G.O.F.B. and S.G.W. conducted protein extraction, ELISA and purification experiments. S.L.W., J.L.J., Z.L.N., and K.K.M. supervised the study and reviewed the
manuscript. CORRESPONDING AUTHORS Correspondence to Mona B. Damaj or Kranthi K. Mandadi. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL
INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION
SUPPLEMENTARY INFORMATION. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation,
distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and
indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to
the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will
need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE
CITE THIS ARTICLE Damaj, M.B., Jifon, J.L., Woodard, S.L. _et al._ Unprecedented enhancement of recombinant protein production in sugarcane culms using a combinatorial promoter stacking
system. _Sci Rep_ 10, 13713 (2020). https://doi.org/10.1038/s41598-020-70530-z Download citation * Received: 06 December 2019 * Accepted: 21 July 2020 * Published: 13 August 2020 * DOI:
https://doi.org/10.1038/s41598-020-70530-z SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative