Non-alcoholic fatty liver disease is associated with bacterial translocation and a higher inflammation response in psoriatic patients
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Psoriasis and non-alcoholic fatty liver disease (NAFLD) are both inflammatory diseases. The study objective was to estimate the risk of NAFLD, non-alcoholic steatohepatitis, and
liver fibrosis (by liver stiffness and liver biopsy) in patients with psoriasis and to determine the epidemiological, clinical, immunological (TNF-α, IL-2, IL-6, IL-12, IL-17, IL-23, and
TGF-β) characteristics, and bacterial translocation. Of the 215 psoriatic patients included, 91 presented NAFLD (prevalence: 42.3%). Compared to patients with psoriasis alone, those with
NAFLD were significantly more likely to have metabolic syndrome, diabetes, dyslipidemia, body mass index ≥ 30 kg/m2, homeostatic model assessment of insulin resistance ≥ 2.15, and greater
psoriasis area severity index. NAFLD patients also had significantly higher levels of TNF-α (p = 0.002) and TGF-β (p = 0.007) and a higher prevalence of bacterial translocation (29.7% vs.
13.7%; p = 0.004). Liver stiffness measurement was over 7.8 kPa in 17.2% (15/87) of NAFLD patients; 13 of these underwent liver biopsy, and 5.7% (5/87) had liver fibrosis, while 1.1% (1/87)
had advanced fibrosis or non-alcoholic steatohepatitis. In conclusion the prevalence of NAFLD in patients with psoriasis is high and associated with a higher prevalence of metabolic syndrome
features, bacterial translocation and a higher pro-inflammatory state. It is worth mentioning that liver fibrosis and non-alcoholic steatohepatitis are not frequent in this population of
patients. SIMILAR CONTENT BEING VIEWED BY OTHERS A CROSS SECTIONAL STUDY ASSESSING STEATOTIC LIVER DISEASE IN PATIENTS WITH SYSTEMIC LUPUS ERYTHEMATOSUS Article Open access 20 June 2024
METABOLIC DYSFUNCTION-ASSOCIATED FATTY LIVER DISEASE IN PEOPLE LIVING WITH HIV Article Open access 06 June 2023 THE ADDITIVE EFFECT OF GENETIC AND METABOLIC FACTORS IN THE PATHOGENESIS OF
NONALCOHOLIC FATTY LIVER DISEASE Article Open access 20 October 2022 INTRODUCTION Psoriasis is a systemic, immune-mediated genetic disease associated with several comorbidities (particularly
in severe forms)1,2,3,4,5; indeed, over the last years several studies have been shown that non-alcoholic fatty liver disease (NAFLD) to be highly prevalent in patients with psoriasis6,7.
The term NAFLD encompasses a wide spectrum of hepatic lesions, ranging from simple fatty liver to non-alcoholic steatohepatitis (NASH), and including variable degrees of liver fibrosis,
cirrhosis, and even hepatocellular carcinoma8,9. The global prevalence of NAFLD in the general population is estimated to be 25%10, and it is currently one of the leading causes of cirrhosis
and liver transplantation11. Nowadays, NAFLD is a growing epidemic, in part due to obesity, insulin resistance, and metabolic syndrome12, but also due to psoriasis13. It is striking that
the same comorbidities, especially those associated with metabolic disorders that can promote liver steatosis, have been associated with systemic inflammation in psoriasis. Moreover,
specific proinflammatory mediators have been shown to cause a chronic inflammatory state in NAFLD, psoriasis, and metabolic syndrome14,15. These similarities could indicate a linked
pathogenesis between psoriasis and NAFLD, with a potentially increased risk for advanced hepatic disease12. However, controversy remains around whether the chronic inflammatory nature of
psoriasis is a contributing factor or an independent risk factor for the development of NAFLD. Dysregulation of immune responses in individuals who are genetically susceptible to psoriasis
and have been exposed to an external environmental trigger is essential in plaque psoriasis pathogenesis16,17,18. Various cytokines (tumor necrosis factor [TNF]-α, type I interferons [IFNs],
interleukin [IL]-12, IFN-γ, IL-23, IL-17, and IL-22) mediate the interaction of keratinocytes, dendritic cells, T cells, and other immune cells, causing an abnormal loop proliferation of
keratinocytes in the psoriatic epidermis15. Cytokines are elements of immunity that mediate the inflammatory response of the psoriasis plaque and play a mechanistic role in the development
of insulin resistance and fatty liver disease. However, their role as biomarkers of NAFLD in psoriatic patients is not well established19,20. Previous studies have suggested that NAFLD is
associated with an increased pro-inflammatory state related to abnormal intestinal permeability, endotoxemia and bacterial translocation21,22, so the NAFLD pro-inflammatory profile could be
associated with psoriasis. The aim of this study was to estimate the risk of NAFLD, NASH, and liver fibrosis in patients with psoriasis and to determine the epidemiological, clinical,
immunological, and inflammatory characteristics, along with the rate of bacterial translocation, in these patients. RESULTS An initial sample of 309 patients with moderate to severe
psoriasis were registered during the recruitment period. After excluding 79 patients who presented at least one exclusion criterion, 13 who did not complete the required testing, and 2 who
did not have available cytokines, we finally included 215 patients (120 men and 95 women; Supplementary Fig. S1). PREVALENCE AND RISK FACTORS OF NAFLD Ninety-one (42.3%) included patients
presented NAFLD: 62 cases were mild, and 29 were moderate to severe. Patients with and without NAFLD were similar in terms of the treatments they were receiving for psoriasis, except for
acitretin, which was more common in patients with NAFLD (11.0% vs 3.2%; p = 0.02; Supplementary Table S1). NAFLD was associated with male sex; older age; body mass index (BMI) of 30 kg/m2 or
more; homeostatic model assessment of insulin resistance (HOMA-IR) of more than 2.15; diabetes mellitus; cardiopathy; dyslipidemia; metabolic syndrome; higher waist circumference, levels of
aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyl transferase (GGT), LDL-cholesterol, triglycerides, erythrocyte sedimentation rate (ERS), and
high-sensitivity C-reactive protein (hs-CRP); and non-viable bacterial translocation (BT). Smoking was more prevalent in psoriatic patients without NAFLD. Patients with psoriatic arthritis
did not present more NAFLD (Table 1). Multivariable logistic regression analysis confirmed that HOMA-IR over 2.15 (p < 0.001), male sex (p = 0.005) and BMI of 30 kg/m2 or more (p =
0.007), AST more than 32 U/L (p = 0.036), and triglycerides of more than 150 mg/dL (p = 0.026) significantly increased the risk of NAFLD (Table 2). Comparing mild and moderate-to-severe
NAFLD cases, significant differences were found in age; high BMI; prevalence of smoking, metabolic syndrome, and non-viable BT; and abnormal AST and ALT (Supplementary Table S2). CORRELATION
OF CLINICAL SEVERITY SCALES OF PSORIASIS AND NAFLD Patients with both psoriasis and NAFLD presented higher absolute psoriasis area severity index (PASI), body surface area (BSA), and
physician’s global assessment (PGA) (p < 0.01) than those with psoriasis alone. These groups were similar in terms of psoriasis symptoms and quality of life (Table 2). PROFILE OF
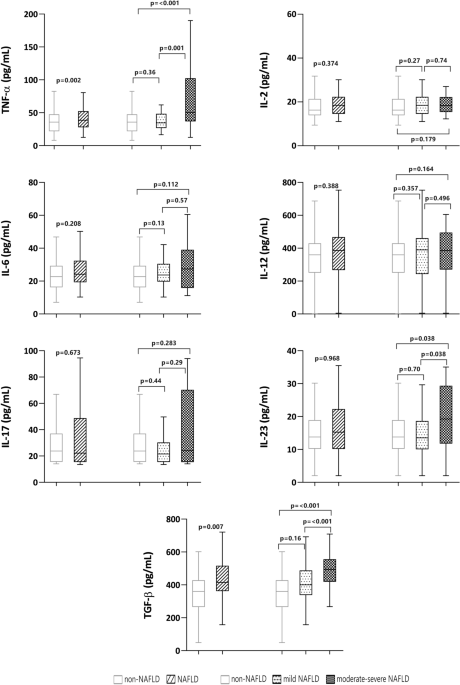
CYTOKINES AND NAFLD Psoriatic patients with NAFLD had significantly higher levels of TNF-α (p = 0.002), transforming growth factor-beta (TGF-β) (p = 0.007), and IL-23 (p < 0.001) than
those without NAFLD (Fig. 1). In the receiver operating characteristic (ROC) analysis, only TNF-α and TGF-β reached statistical significance for the discrimination of NAFLD, with area under
curve (AUC) values of 0.64 and 0.68, respectively, using the following cutoffs: TNF-α, 25.48 pg/mL and TGF-β, 382.6 pg/mL (Fig. 2). Sensitivity and specificity are shown in Supplementary
Table S3. In an exploratory multivariable analysis, testing associations with NAFLD, we introduced TNF-α, TGF-β, age, sex, HOMA-IR ≥ 2.15, and BMI ≥ 30 kg/m2 into the logistic regression
model. We observed independent associations between NAFLD and age (adjusted odds ratio [OR] 1.04; 95% coefficient interval [CI] 1.01–1.05; p = 0.005), male sex (adjusted OR 2.79; 95% CI
1.34–5,83; p = 0.006), values of TNF-α ≥ 20.6 pg/mL (adjusted OR 3.11; 95% CI 1.29–7.63; p = 0.011); BMI ≥ 30 kg/m2 (adjusted OR 3.63; 95% CI 1.76–7.49; pa0.001; and HOMA-IR ≥ 2.15 (adjusted
OR 6.49; 95% CI 2.56–16.43; p < 0.001). In this model, the p value for the Hosmer–Lemeshow goodness-of-fit test was 0.75, and the AUC of 0.79 indicated good predictive ability. PROFILE
OF CYTOKINES AND NON-VIABLE BT Among the 215 patients studied, 44 had non-viable BT (20.6%). These patients had significantly (p < 0.01) higher levels of TNF-α, IL-6, IL-17, IL-23 and
TGF-β than those without BT, regardless of whether or not they had NAFLD. However, BT was more prevalent in patients with NAFLD (29.7% vs 13.7%; p < 0.001), who had higher values of
TNF-α, Il-6, IL-17, IL-23, and TGF-β than those with NAFLD but without BT (p < 0.001). In contrast, patients without NAFLD and with BT only had significantly elevated TNF-α, IL-17, and
IL-23 compared to those without BT (p < 0.001) (Fig. 3). NASH AND LIVER FIBROSIS IN PSORIATIC PATIENTS WITH NAFLD Liver stiffness measurement (LSM) by transient elastography was performed
in 87 of the 91 patients with NAFLD. Fifteen (17.2%) had an LSM of more than 7.8 kPa, suggesting a high risk of liver fibrosis; 13 of these patients underwent liver biopsy to assess liver
fibrosis and NASH (Table 3). NASH was diagnosed in only one. Five of the 87 (5.7%) patients with NAFLD had liver fibrosis confirmed by histology; however, only one patient had advanced liver
fibrosis (F3). A higher proportion of patients with NAFLD and a high risk of fibrosis according to LSM had diabetes mellitus, BMI over 30 kg/m2, ALT over 33 UI, and ERS over 16 mm, compared
to patients with a low risk of fibrosis (Supplementary Table S4). Multivariable logistic regression analysis confirmed this association for diabetes mellitus and high ALT and ERS
(Supplementary Table S5). Patients with high and low LSM had a similar cytokines profile (Supplementary Table S6). DISCUSSION This study shows a prevalence of NAFLD of 42.3% in psoriatic
patients—higher than the prevalence reported in the general population in Spain (25.8% to 33.4% in men and 20.3% in women)14,23, and consistent with other prevalence studies in people with
psoriasis (44% to 65.6%)6,7,15,24,25. The pathogenesis of psoriasis comorbidities, including NAFLD, is not completely understood. Different factors are known to be involved, including common
patterns of immune responses and inflammatory pathways, genetic predisposition, and shared risk factors5. These risk factors are largely similar to those in the general population and are
related to metabolic syndrome26,27. In our patients, NAFLD was independently related to male sex, age, and metabolic syndrome comorbidities. These results are in line with previous studies
linking metabolic syndrome with NAFLD28,29, which could be related to insulin resistance as a shared pathogenic pathway in both NAFLD and metabolic syndrome in people with psoriasis. In
keeping with previous reports7,25,27,30, our patients with NAFLD had a greater PASI compared to those without. However, severity scores in our patients were generally low because most were
under systemic therapy, so caution is warranted when interpreting this result. When analyzing specific psoriasis treatments, only acitretin appeared to be associated with NAFLD; however,
other studies have not detected any relationship25,31,32. Patients on methotrexate did not show an increased risk of liver steatosis, in agreement with previous studies27,33. This finding is
notable since methotrexate is frequently used for treating psoriasis but is involved in the development of liver fibrosis34, especially when used long-term in patients with other risk
factors for fatty liver disease, like excessive alcohol use, obesity, and diabetes35. However, in single dose regimens of methotrexate 5 mg to 15 mg weekly, plus folate supplementation,
fibrosis and clinically apparent liver disease are rare even with long-term use, as observed in our study36. According to ultrasound-based grading of NAFLD severity, participants with
moderate-to-severe NAFLD were older and more obese, and they showed more pronounced abnormalities in their metabolic profile, in line with other studies37. Moreover, these patients had a
higher level of transaminases and non-viable BT, probably related to increased shared inflammatory status, which in turn could be linked to psoriasis activity at the time of the study.
Patients with NAFLD also presented greater inflammatory activity, as determined by the cytokines TNF-α and TGF-β, and this activity was greater in patients with moderate-to-severe NAFLD.
Pro-inflammatory cytokines, including TNF-α, play a crucial role in pathogenesis of both NAFLD and psoriasis, as well as in the progression of NAFLD to non-alcoholic steatohepatitis (NASH).
Indeed, psoriasis-related inflammation could trigger the development of NAFLD2. Previous studies have shown that bacterial DNA can translocate to extra-intestinal sites and promote an
immunological response similar to that produced by viable bacteria, giving rise to an inflammatory state that can complicate chronic liver disease38,39. Mechanisms proposed to promote BT
include increased gut permeability, intestinal bacterial overgrowth, and alterations in the gut microbiota composition40,41,42,43. This is added a with genetic predisposition to BT and
inflammation as seen in other clinical situation as Crohn's disease33,44. So a new line of research is opened on alterations in genes related to the inflammatory response as an
additional pathogenic mechanism in psoriasis BactDNA in the bloodstream that promotes an immunological response, favoring the release of pro-inflammatory cytokines and perpetuating chronic
inflammation42. Furthermore, the synthesis of cytokines, mainly TNF-α, increases intestinal permeability, probably bringing on BT from the intestinal lumen to the bloodstream45, and it has
been associated with inflammatory conditions, such as inflammatory bowel disease42, and maybe psoriasis. Ramirez et al.46 suggest a role for BT in active plaque psoriasis, which is more
evident in patients with longer duration and earlier onset of the disease. Additionally, our results indicate that BT is more frequent in psoriatic patients with versus without NAFLD. This
finding may be of interest since one of the factors in the development and progression of NAFLD is gut permeability, which may be mediated by the microbiome. Although the role of the
BT-measured microbiome in patients with psoriasis and NAFLD has not been evaluated so far, in our study BT was associated with a higher estimated inflammatory response (elevation of
proinflammatory cytokines TNF-α and TGF-β) in patients with versus without NAFLD. While patients with both psoriasis and NAFLD have an increased inflammatory response, liver damage, as
estimated by the degree of liver fibrosis, was modest in our cohort. Only 5.7% of patients in the total cohort had liver fibrosis on liver biopsy, and only one patient met histological
criteria for the diagnosis of NASH. To our knowledge, ours is one of the few published studies that have assessed NASH and liver fibrosis by liver biopsy in patients with psoriasis. In
another, the prevalence of NASH in psoriatic patients was about 22%24. The low rate of NASH in our series may be partly due to the fact that 68% of NAFLD patients were under systemic
treatment, which could modulate the inflammatory response in the liver. Patients at high risk of fibrosis, as estimated by LSM, had a higher prevalence of diabetes, obesity, and elevated
transaminases. This association is explained by the important role that both diabetes and metabolic syndrome play in the pathogenesis of liver fibrosis in NAFLD patients47. This study has
the limitations inherent in all single-center studies, and the results cannot be extrapolated to a population outside the Mediterranean region, where exposure to sunshine and dietary habits
may differ. Moreover, only a small number of patients with elastography values of over 7.8 kPa underwent liver biopsy, which may explain the absence of any observed relationship between
fibrosis and clinical and analytical parameters. Another limitation, related to the real-life study design, is the inclusion of patients with very heterogeneous characteristics (naïve
patients, in systemic treatment or not, phototherapy, topical treatment). Thus, the severity of psoriasis, as measured by PASI, BSA, and PGA, was low at the time of the study, which could
affect the interpretation of the relationship that disease severity has with comorbidities and inflammatory parameters. However, the heterogenous sample could also be considered a strength,
as data were obtained from a large population in routine clinical practice. Another strength of the study is its prospective and protocolized nature, ensuring standardized procedures by the
research team. In conclusion, approximately 40% of our patients with moderate-to-severe psoriasis had NAFLD, and this was more common in older, obese men with more severe psoriasis and
metabolic syndrome. Psoriatic patients with NAFLD—especially older, obese men with higher HOMA-IR and moderate-to-severe NAFLD—were more likely to have non-viable BT and higher levels of
TNF-α and TGF-β. Up to 10% of NAFLD patients showed LSM suggesting a high risk of liver fibrosis, but this condition was confirmed by histology in just 5.7% of the patients. Diabetes
mellitus, elevated ALT and ERS were more common in patients at high risk of fibrosis. Our study suggests that chronic inflammation, represented by higher levels of TNF-α and TGF-β, and
non-viable BT could contribute to the development of NAFLD in patients with psoriasis. However, our data are descriptive and do not provide mechanistic evidence to definitively demonstrate
this. Additional experimental studies are needed to evaluate the underlying biological mechanisms and confirm this pathogenic association. MATERIAL AND METHODS DESIGN AND SETTING This
prospective, descriptive observational study took place at the psoriasis unit of the dermatology service in the General University Hospital of Alicante, located on the south-eastern
Mediterranean coast of Spain. This unit serves a population of approximately 267,000 people. PARTICIPANTS AND DATA COLLECTION Inclusion criteria were: aged at least 18 years, with a
diagnosis of moderate-to-severe psoriasis48, , presenting to our center from 1 September 2017 to 31 May 2018, and providing informed consent. Exclusion criteria along with epidemiological,
clinical, and laboratory variables are recorded in Supplementary Table S7. Metabolic syndrome was defined according to the National Cholesterol Education Program Adult Treatment Panel III49.
Analytical parameters were dichotomized according to the thresholds for normal values used in our laboratory50. Active treatment was defined as that which started at least six months
before, and was classified into five categories: topical treatment, phototherapy, systemic treatment (methotrexate, retinoids, and cyclosporine), apremilast and biologics (including both
anti-TNF, anti-IL-12/23, anti-IL-17, and anti-IL-23 therapies). Severity was determined according to the BSA affected, PASI, and PGA (range 0 to 4). Visual analog scales were employed to
measure pain and itch related to psoriasis, and the DLQI to assess quality of life. IDENTIFICATION OF NON-VIABLE BACTERIAL TRANSLOCATION Non-viable BT was defined as the presence of bactDNA
in blood in a negative microbiological culture. Total DNA extraction from homogenized specimens was undertaken with the QIAamp DNA Tissue kit (QIAgen, Barcelona, Spain). A standard PCR
followed by partial nucleotide sequencing of the 16SrRNA gene was performed according to the methodology described elsewhere51. Two microliters of template were added into a reaction mix
containing 10 mmol/L Tris buffer (pH 8.3), 50 mmol/L KCl, 1.5 mmol/L Mg2, 200 μmol/L of each deoxynucleoside triphosphate, 50 pmol of primers 5′-AGAGTTTGAT-CATGGCTCAG-3′ and
5′-ACCGCGACTGCTGCT-GGCAC-3′, and 1.25 U BioTaq (Bioline, London, England) to reach a final volume of 50 μL. The primers located at positions 7–27 and 531–514 (_Escherichia coli_ numbering)
are universal eubacterial primers that will amplify any known bacterial 16S ribosomal RNA gene. A 35-cycle PCR was run in a GeneAmp 9700 (Applied Bio-systems, Foster City, CA) at: 94 °C for
30 s, 55 °C for 30 s, and 72°. The detection limit of the technique was 5 pg/mL of bacterial DNA. Samples under detection limit were considered negative. MEASURE OF SERUM CYTOKINE LEVELS
Enzyme-linked immunoabsorbent assays (ELISAs) were performed in serum for TNF-α, IL-2, IL-6, IL-17, IL-23, IL-12 and (TGF-β (Rat Quantikine kits, R&D Systems, Minneapolis, MN, USA). All
samples were tested in triplicate and read in a Sunrise Microplate Reader (Tecan, Männedorf, Switzerland). The lower limit of detection for each assay was 5 pg/mL. Standard curves were
generated for every plate, and the average zero standard optical densities were subtracted from the rest of the standards, controls, and samples to obtain a corrected concentration.
ASSESSMENT OF NAFLD A board-certified radiologist performed hepatic ultrasound in all participants, using a Toshiba Aplio 300 and Aplio 500 ultrasound scanner with a 3.5-MHz Convex abdominal
probe. All patients included underwent a hepatic ultrasound to assess hepatic steatosis, which was defined by unique features including bright hepatic echoes, increased hepatorenal
echogenicity, and vascular blurring of the portal or hepatic vein. Severity was defined as grade 1 (mild steatosis), 2 (moderate), or 3 (severe steatosis), as described elsewhere52. NAFLD
was diagnosed in patients with hepatic steatosis after excluding secondary causes of steatosis. We classified NAFLD patients in two groups based on the severity of steatosis, as assessed by
ultrasound: mild NAFLD and moderate-to-severe NAFLD. Non-invasive tests for liver fibrosis were performed in all NAFLD patients: NAFLD fibrosis score (NFS), Fibrosis-4 (FIB-4), and hepatic
transient elastography by Fibroscan, as described elsewhere53. Patients with LSM of more than 7.8 kPa underwent liver biopsy to confirm advanced liver fibrosis and/or NASH. Liver biopsies
were performed percutaneously with ultrasound guidance and 16 G × 15 cm tru-cut needles (Biopince Full core Biopsy Instrument; Argon medical Devices, TX USA). Liver histology was classified
according to the NAFLD activity score (NAS)54. NASH was defined as steatosis plus lobular inflammation and ballooning degeneration55. STATISTICAL ANALYSIS Descriptive statistics were used to
summarize the data. Categorical variables were compared according to the presence of NAFLD and explanatory variables including age, sex, and metabolic syndrome, using the chi-squared test
of homogeneity. We undertook a ROC curve analysis, establishing a cutoff for each of the cytokines to identify individuals who had NAFLD. Crude ORs and 95% CIs were calculated to assess the
association between explanatory variables and both NAFLD and risk of fibrosis. All variables yielding a p value of less than 0.05 were included in a saturated, multivariable logistic
regression model. All analyses were carried out IBM SPSS Statistics v25 for Mac (Armonk, NY) or Prism (GraphPad). ETHICAL ASPECTS The institutional review board of University General
Hospital of Alicante & Alicante Institute of Sanitary and Biomedical Research (ISABIAL), reviewed and approved the study protocol (Ref. CEIC PI2017/27). All participants provided written
informed consent. Data confidentiality and patient anonymity were maintained at all times, in accordance with Spanish regulations on observational studies56. ABBREVIATIONS * ALT: Alanine
aminotransferase * AST: Aspartate aminotransferase * AUC: Area under curve * BAS: Body surface area * BMI: Body mass index * BT: Bacterial translocation * DLQI: Dermatology life quality
index * ELISA: Enzyme-linked immunoabsorbent assays * ESR: Erythrocyte sedimentation rate * GGT: Gamma-glutamyl transferase * FIB-4: Fibrosis-4 * HOMA-IR: Homeostatic model assessment of
insulin resistance * hs-CRP: High-sensitivity C-reactive protein * IFN: Interferon * IL: Interleukin * LSM: Liver stiffness measurement * NAFLD: Non-alcoholic fatty liver disease * NAS:
NAFLD activity score * NASH: Non-alcoholic steatohepatitis * NFS: NAFLD fibrosis score * OD: Odds ratio * PASI: Psoriasis area severity index * PCR: Polymerase chain reaction * PGA:
Physician’s global assessment * ROC: Receiver operating characteristic * TGF: Transforming growth factor * TNF: Tumor necrosis factor REFERENCES * Dauden, E. _et al._ Position statement for
the management of comorbidities in psoriasis. _J. Eur. Acad. Dermatol. Venereol._ 32, 2058–2073 (2018). Article CAS PubMed Google Scholar * Mantovani, A., Gisondi, P., Lonardo, A. &
Targher, G. Relationship between non-alcoholic fatty liver disease and psoriasis: A novel Hepato-Dermal axis?. _Int. J. Mol. Sci._ 17, 217 (2016). Article PubMed PubMed Central CAS
Google Scholar * Griffiths, C. E. & Barker, J. N. Pathogenesis and clinical features of psoriasis. _Lancet_ 370, 263–271 (2007). Article CAS PubMed Google Scholar * Ferrándiz, C.,
Carrascosa, J. M. & Toro, M. Prevalencia de la psoriasis en España en la era de los agentes biológicos. _Actas Dermosifiliogr._ 105, 504–509 (2014). Article PubMed Google Scholar *
Takeshita, J. _et al._ Psoriasis and comorbid diseases: Epidemiology. _J. Am. Acad. Dermatol._ 76, 377–390 (2017). Article PubMed PubMed Central Google Scholar * Gisondi, P., Targher,
G., Zoppini, G. & Girolomoni, G. Non-alcoholic fatty liver disease in patients with chronic plaque psoriasis. _J. Hepatol._ 51, 758–764 (2009). Article CAS PubMed Google Scholar *
Abedini, R., Salehi, M., Lajevardi, V. & Beygi, S. Patients with psoriasis are at a higher risk of developing nonalcoholic fatty liver disease. _Clin. Exp. Dermatol._ 40, 722–727 (2015).
Article CAS PubMed Google Scholar * Wenk, K. S., Arrington, K. C. & Ehrlich, A. Psoriasis and non-alcoholic fatty liver disease. _J. Eur. Acad. Dermatol. Venereol._ 25, 383–391
(2011). Article CAS PubMed Google Scholar * Musso, G., Gambino, R., Cassader, M. & Pagano, G. Meta-analysis: Natural history of non-alcoholic fatty liver disease (NAFLD) and
diagnostic accuracy of non-invasive tests for liver disease severity. _Ann. Med._ 43, 617–649 (2011). Article PubMed Google Scholar * Younossi, Z. _et al._ Global burden of NAFLD and
NASH: Trends, predictions, risk factors and prevention. _Nat. Rev. Gastroenterol. Hepatol._ 15, 11–20 (2018). Article PubMed Google Scholar * Pais, R. _et al._ NAFLD and liver
transplantation: Current burden and expected challenges. _J. Hepatol._ 65, 1245–1257 (2016). Article PubMed PubMed Central Google Scholar * Awosika, O. _et al._ A case-control study to
evaluate the prevalence of nonalcoholic fatty liver disease among patients with moderate-to-severe psoriasis. _J. Clin. Aesthet. Dermatol._ 11, 33–37 (2018). PubMed PubMed Central Google
Scholar * Icen, M. _et al._ Trends in incidence of adult-onset psoriasis over three decades: A population-based study. _J. Am. Acad. Dermatol._ 60, 394–401 (2009). Article PubMed PubMed
Central Google Scholar * Phan, K., Onggo, J., Charlton, O. & Smith, S. D. Relationship between psoriasis and non-alcoholic fatty liver disease: Updated systematic review and adjusted
meta-analysis. _Australas. J. Dermatol._ 60, e352–e355 (2019). Article PubMed Google Scholar * Gisondi, P., Bellinato, F., Girolomoni, G. & Albanesi, C. Pathogenesis of chronic plaque
psoriasis and its intersection with cardio-metabolic comorbidities. _Front. Pharmacol._ 11, 12 (2020). Article CAS Google Scholar * Perera, G. K., Di Meglio, P. & Nestle, F. O.
Psoriasis. _Annu. Rev. Pathol. Mech. Dis._ 7, 385–422 (2012). Article CAS Google Scholar * Engler, D., Chezuba, H. P. & Masuku, P. Psoriasis. _SA Pharm. J._ 84, 38–42 (2017). Google
Scholar * Frank Nestle, P. O., Kaplan, D. H. & Barker, J. Psoriasis. _N. Engl. J. Med._ 361, 496–505 (2009). Article PubMed Google Scholar * Muramatsu, S., Kubo, R., Nishida, E.
& Morita, A. Serum interleukin-6 levels in response to biologic treatment in patients with psoriasis. _Mod. Rheumatol._ 27, 137–141 (2017). Article CAS PubMed Google Scholar * Bai,
F. _et al._ Serum levels of adipokines and cytokines in psoriasis patients: A systematic review and meta-analysis. _Oncotarget_ 9, 1266–1278 (2018). Article PubMed Google Scholar * Miele,
L. _et al._ Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. _Hepatology_ 49, 1877–1887 (2009). Article CAS PubMed Google Scholar *
Henao-Mejia, J. _et al._ Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. _Nature_ 482, 179–185 (2012). Article ADS CAS PubMed PubMed Central Google Scholar
* Caballería, L. _et al._ Prevalence and factors associated with the presence of nonalcoholic fatty liver disease in an adult population in Spain. _Eur. J. Gastroenterol. Hepatol._ 22, 24–32
(2010). Article PubMed CAS Google Scholar * Roberts, K. K. _et al._ The prevalence of NAFLD and NASH among patients with psoriasis in a tertiary care dermatology and rheumatology
clinic. _Aliment. Pharmacol. Ther._ 41, 293–300 (2015). Article CAS PubMed Google Scholar * Magdaleno-Tapial, J. _et al._ Prevalence of non-alcoholic fatty liver and liver fibrosis in
patients with moderate-severe psoriasis: A cross-sectional cohort study. _Australas. J. Dermatol._ 61, 105–109 (2020). Article PubMed Google Scholar * Caballería, L. _et al._ Metabolic
syndrome and nonalcoholic fatty liver disease in a Spanish population: Influence of the diagnostic criteria used. _Eur. J. Gastroenterol. Hepatol._ 24, 1007–1011 (2012). Article PubMed
Google Scholar * Caballería, L. _et al._ Factores de riesgo asociados a la presencia de hígado graso no alcohólico: Un estudio de casos y controles. _Med. Clin._ 141, 233–239 (2013).
Article Google Scholar * Younossi, Z. M. _et al._ Global epidemiology of nonalcoholic fatty liver disease: Meta-analytic assessment of prevalence, incidence, and outcomes. _Hepatology_ 64,
73–84 (2016). Article PubMed Google Scholar * Love, T. J., Qureshi, A. A., Karlson, E. W., Gelfand, J. M. & Choi, H. K. Prevalence of the metabolic syndrome in psoriasis: Results
from the national health and nutrition examination survey, 2003–2006. _Arch. Dermatol._ 147, 419–424 (2011). Article PubMed Google Scholar * Ramos, A. N., de OliveiraRocha, B., de
AlmeidaRêgo, V. R. & de Follador, O. M. The linkage between psoriasis and non-alcoholic fatty liver disease: a literature review: PubMed. _Acta Dermatovenerol. Croat_ 22, 132–136 (2014).
PubMed Google Scholar * Miele, L. _et al._ Prevalence, characteristics and severity of non-alcoholic fatty liver disease in patients with chronic plaque psoriasis. _J. Hepatol._ 51,
778–786 (2009). Article CAS PubMed Google Scholar * Chularojanamontri, L., Silpa-archa, N., Wongpraparut, C. & Limphoka, P. Long-term safety and drug survival of acitretin in
psoriasis: a retrospective observational study. _Int. J. Dermatol._ 58, 593–599 (2019). Article CAS PubMed Google Scholar * Pongpit, J. _et al._ Liver stiffness measurement in psoriasis:
Do metabolic or disease factors play the important role?. _Biomed Res. Int._ 2016, 1–6 (2016). Article CAS Google Scholar * Carrascosa, J. M., Bonanad, C., Dauden, E., Botella, R. &
Olveira-Martín, A. Psoriasis and nonalcoholic fatty liver disease. _Actas Dermo-Sifiliograficas_ 108, 506–514 (2017). Article CAS PubMed Google Scholar * Rosenberg, P. _et al._ Psoriasis
patients with diabetes type 2 are at high risk of developing liver fibrosis during methotrexate treatment. _J. Hepatol._ 46, 1111–1118 (2007). Article CAS PubMed Google Scholar *
Walker, A. M. _et al._ Determinants of serious liver disease among patients receiving low-dose methotrexate for rheumatoid arthritis. _Arthritis Rheum._ 36, 329–335 (1993). Article CAS
PubMed Google Scholar * Cuenza, L. R., Razon, T. L. J. & Dayrit, J. C. Correlation between severity of ultrasonographic nonalcoholic fatty liver disease and cardiometabolic risk among
Filipino wellness patients. _J. Cardiovasc. Thorac. Res._ 9, 85–89 (2017). Article PubMed PubMed Central Google Scholar * Francés, R. _et al._ Translocation of bacterial DNA from
Gram-positive microorganisms is associated with a species-specific inflammatory response in serum and ascitic fluid of patients with cirrhosis. _Clin. Exp. Immunol._ 150, 230–237 (2007).
Article PubMed PubMed Central CAS Google Scholar * Bellot, P., Francés, R. & Such, J. Pathological bacterial translocation in cirrhosis: Pathophysiology, diagnosis and clinical
implications. _Liver Int._ 33, 31–39 (2013). Article CAS PubMed Google Scholar * Gutiérrez, A. _et al._ Gut bacterial DNA translocation is an independent risk factor of flare at short
term in patients with crohn’s disease. _Am. J. Gastroenterol._ 111, 529–540 (2016). Article ADS PubMed CAS Google Scholar * Gutiérrez, A. _et al._ Antimicrobial peptide response to
blood translocation of bacterial DNA in Crohn’s disease is affected by NOD2/CARD15 genotype. _Inflamm. Bowel Dis._ 17, 1641–1650 (2011). Article PubMed Google Scholar * Gutiérrez, A. _et
al._ Cytokine association with bacterial DNA in serum of patients with inflammatory bowel disease. _Inflamm. Bowel Dis._ 15, 508–514 (2009). Article PubMed Google Scholar * Gómez-Hurtado,
I. _et al._ Norfloxacin is more effective than Rifaximin in avoiding bacterial translocation in an animal model of cirrhosis. _Liver Int._ 38, 295–302 (2018). Article PubMed CAS Google
Scholar * Gutiérrez, A. _et al._ Genetic susceptibility to increased bacterial translocation influences the response to biological therapy in patients with Crohn’s disease. _Gut_ 63,
272–280 (2014). Article PubMed CAS Google Scholar * Hispán, P. _et al._ Identification of bacterial DNA in the peripheral blood of patients with active hidradenitis suppurativa. _Arch.
Dermatol. Res._ 312, 159–163 (2020). Article PubMed CAS Google Scholar * Ramírez-Boscá, A. _et al._ Identification of bacterial DNA in the peripheral blood of patientswith active
psoriasis. _JAMA Dermatol._ 151, 670–671 (2015). Article PubMed Google Scholar * Dixon, J. B., Bhathal, P. S. & O’Brien, P. E. Nonalcoholic fatty liver disease: Predictors of
nonalcoholic steatohepatitis and liver fibrosis in the severely obese. _Gastroenterology_ 121, 91–100 (2001). Article CAS PubMed Google Scholar * Daudén, E., Puig, L., Ferrándiz, C.,
Sánchez-Carazo, J. L. & Hernanz-Hermosa, J. M. Consensus document on the evaluation and treatment of moderate-to-severe psoriasis: Psoriasis Group of the Spanish Academy of Dermatology
and Venereology. _J. Eur. Acad. Dermatol. Venereol._ 30, 1–18 (2016). Article PubMed Google Scholar * Cleeman, J. I. Executive summary of the third report of the National Cholesterol
Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). _J. Am. Med. Assoc._ 285, 2486–2497 (2001).
Article Google Scholar * Lee, J. H. _et al._ Hepatic steatosis index: A simple screening tool reflecting nonalcoholic fatty liver disease. _Dig. Liver Dis._ 42, 503–508 (2010). Article
CAS PubMed Google Scholar * Such, J. _et al._ Detection and identification of bacterial DNA in patients with cirrhosis and culture-negative, nonneutrocytic ascites. _Hepatology_ 36,
135–141 (2002). Article CAS PubMed Google Scholar * Dasarathy, S. _et al._ Validity of real time ultrasound in the diagnosis of hepatic steatosis: A prospective study. _J. Hepatol._ 51,
1061–1067 (2009). Article PubMed PubMed Central Google Scholar * Anstee, Q. M. _et al._ Noninvasive tests accurately identify advanced fibrosis due to NASH: Baseline data from the
STELLAR trials. _Hepatology_ 70, 1521–1530 (2019). Article PubMed Google Scholar * Kleiner, D. E. _et al._ Design and validation of a histological scoring system for nonalcoholic fatty
liver disease. _Hepatology_ 41, 1313–1321 (2005). Article PubMed Google Scholar * Yeh, M. M. & Brunt, E. M. Pathological features of fatty liver disease. _Gastroenterology_ 147,
754–764 (2014). Article CAS PubMed Google Scholar * AHRQ. _Quality Indicators. Prevention Quality Indicators Technical Specifications Updates—Version 6.0 (ICD-9)_.
https://www.qualityindicators.ahrq.gov/Archive/PQI_TechSpec_ICD09_v60.aspx (2016). Download references ACKNOWLEDGEMENTS This research has been supported by a research grant from the
Institute of Health and Biomedical Research of Alicante (ISABIAL)/FISABIO Foundation (180140), the Innovation Prize in the Health Field of Celgene of the University of Alcalá de Henares, and
an unrestricted grant by Abbvie. We would like to thank Meggan Harris for her help in editing the manuscript. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Dermatology Department,
University General Hospital of Alicante & Alicante Institute of Sanitary and Biomedical Research (ISABIAL), C/Pintor Baeza, 12, 03010, Alicante, Spain Isabel Belinchón-Romero * Clinical
Medicine Department, Miguel Hernández University of Elche, Elche, Spain Isabel Belinchón-Romero, Rubén Frances & José-Manuel Ramos-Rincón * Digestive Medicine Department, University
General Hospital of Alicante & Alicante Institute of Sanitary and Biomedical Research (ISABIAL), Alicante, Spain Pablo Bellot * Dermatology Unit, Hospital Quirón-Salud, Tenerife, Spain
David Romero-Pérez * Radiodiagnostic Department, University General Hospital of Alicante & Alicante Institute of Sanitary and Biomedical Research (ISABIAL), Alicante, Spain Isolina
Herraiz-Romero * Immunology Department, University General Hospital of Alicante & Alicante Institute of Sanitary and Biomedical Research (ISABIAL), Alicante, Spain Francisco Marco *
Research Institute, University General Hospital of Alicante & Alicante Institute of Sanitary and Biomedical Research (ISABIAL), Alicante, Spain Rubén Frances * CIBERehd, Instituto de
Salud Carlos III, Madrid, Spain Rubén Frances * Internal Medicine Department, University General Hospital of Alicante-ISABIAL, Alicante, Spain José-Manuel Ramos-Rincón Authors * Isabel
Belinchón-Romero View author publications You can also search for this author inPubMed Google Scholar * Pablo Bellot View author publications You can also search for this author inPubMed
Google Scholar * David Romero-Pérez View author publications You can also search for this author inPubMed Google Scholar * Isolina Herraiz-Romero View author publications You can also search
for this author inPubMed Google Scholar * Francisco Marco View author publications You can also search for this author inPubMed Google Scholar * Rubén Frances View author publications You
can also search for this author inPubMed Google Scholar * José-Manuel Ramos-Rincón View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS I.B.R.
and J.M.R.R planned and designed the project. I.B.R., P.B., D.R.P, I.H.R., F.M., R.F., J.M.R.R. performed the acquisition, analysis and interpretation of data. I.B.R., P.B., and J.M.R.R.
write original draft. All authors reviewed the manuscript. CORRESPONDING AUTHOR Correspondence to Isabel Belinchón-Romero. ETHICS DECLARATIONS COMPETING INTERESTS I.B.R. acted as a
consultant and/or speaker for and/or participated in clinical trials sponsored by companies that manufacture drugs used for the treatment of psoriasis, including Janssen Pharmaceuticals Inc,
Almirall SA, Lilly, AbbVie, Novartis, Celgene, Biogen Amgen, Leo-Pharma, Pfizer-Wyeth, MSD, and UCB. The rest of the authors state do not declare conflicts of interest. ADDITIONAL
INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION
SUPPLEMENTARY INFORMATION. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation,
distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and
indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit
line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use,
you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS
ARTICLE CITE THIS ARTICLE Belinchón-Romero, I., Bellot, P., Romero-Pérez, D. _et al._ Non-alcoholic fatty liver disease is associated with bacterial translocation and a higher inflammation
response in psoriatic patients. _Sci Rep_ 11, 8593 (2021). https://doi.org/10.1038/s41598-021-88043-8 Download citation * Received: 17 January 2021 * Accepted: 06 April 2021 * Published: 21
April 2021 * DOI: https://doi.org/10.1038/s41598-021-88043-8 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative