Unraveling the ecological processes modulating the population structure of escherichia coli in a highly polluted urban stream network
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT _Escherichia coli_ dynamics in urban watersheds are affected by a complex balance among external inputs, niche modulation and genetic variability. To explore the ecological
processes influencing _E. coli_ spatial patterns, we analyzed its abundance and phylogenetic structure in water samples from a stream network with heterogeneous urban infrastructure and
environmental conditions. Our results showed that environmental and infrastructure variables, such as macrophyte coverage, DIN and sewerage density, mostly explained _E. coli_ abundance.
Moreover, main generalist phylogroups A and B1 were found in high proportion, which, together with an observed negative relationship between _E. coli_ abundance and phylogroup diversity,
suggests that their dominance might be due to competitive exclusion. Lower frequency phylogroups were associated with sites of higher ecological disturbance, mainly involving simplified
habitats, higher drainage infrastructure and septic tank density. In addition to the strong negative relationship between phylogroup diversity and dominance, the occurrence of these
phylogroups would be associated with increased facilitated dispersal. Nutrients also contributed to explaining phylogroup distribution. Our study proposes the differential contribution of
distinct ecological processes to the patterns of _E. coli_ in an urban watershed, which is useful for the monitoring and management of fecal pollution. SIMILAR CONTENT BEING VIEWED BY OTHERS
IMPACT OF LAND-USE AND FECAL CONTAMINATION ON _ESCHERICHIA_ POPULATIONS IN ENVIRONMENTAL SAMPLES Article Open access 30 December 2024 INSIGHT INTO IMPACT OF SEWAGE DISCHARGE ON MICROBIAL
DYNAMICS AND PATHOGENICITY IN RIVER ECOSYSTEM Article Open access 27 April 2022 EFFECTS OF HYDROLOGICAL REGIME AND LAND USE ON IN-STREAM _ESCHERICHIA COLI_ CONCENTRATION IN THE MEKONG BASIN,
LAO PDR Article Open access 10 February 2021 INTRODUCTION _Escherichia coli_, one of the most frequently found pathogens in urban waters, has historically been used as a proxy for recent
microbiological pollution, mainly due to its straightforward and low-cost detection methods1. There is consistent evidence that some _E. coli_ strains may persist and even grow in secondary
habitats such as fresh and marine waters and sediments2,3,4,5,6. The ecological processes influencing the population structure of _E. coli_ in urban environments are unclear; its ubiquity
result from a complex balance involving external inputs, ecological conditions and niche processes acting on aquatic habitats, while affecting its intraspecific genetic variability7,8,9.
Therefore, a better understanding of the ecological processes underlying the environmental occurrence of _E. coli_ is a matter of methodological, sanitary and management concern9,10. _E.
coli_ intra-specific variability has been grouped into eight major phylogroups based on genetic analyses: A, B1, B2, C, D, E, F and G11,12,13. Although laboratory and pathogenic _E. coli_
strains have been thoroughly described, the environmental ecological niches associated with phylogroups remain largely unknown. The abundance pattern of each phylogroup seems to vary among
surface freshwater systems, but some of them (A and B1) are consistently predominant and prevalent while others are less frequent and associated to urban areas (e.g., D and F)14,15,16.
According to the coexistence theory, the over-representation of some phylogroups in the environment can be ascribed to competitive fitness differences (e.g., in resource exploitation) that
stabilize their coexistence17. Experimental microcosm studies support the notion that the most abundant _E. coli_ phylogroups show greater capacity of environmental survival. For example,
strains from phylogroup B1 were found to persist longer and to tolerate lower temperatures than the remaining phylogroups18. Moreover, phenotypic analysis revealed that phylogroup B1 strains
were more likely to exhibit traits indicative of a higher ability to colonize aquatic plants (and therefore to persist in freshwater secondary habitats), while A and B2 phenotypes were
linked to an animal-associated lifestyle19. _Escherichia_ strains closely related to _E. coli_ (i.e., _cryptic clades_ I to V), were also associated with long-term environmental
persistence3,20,21. Altogether, this strongly suggests that niche processes such as competitive exclusion and niche partitioning may contribute to shaping the spatial distribution of the
environmental population structure of _E. coli_. However, there is no _in-situ_ evidence supporting the role of niche processes. Several studies focused on bacterial community in urban
streams have revealed the impact of urbanization on population structure, highlighting the need to consider watersheds as part of a complex social-ecological-technological system22,23,24.
Within this framework, ecological modulations may be understood as a balance between local features of the ecosystems, demographic pressures and built-in infrastructure. In this sense, urban
infrastructures, such as stormwater networks and roads, facilitate the transfer of pollutants by surface runoff, acting as links between human activity in land areas of the watershed and
water bodies, turning the latter into sinks25. Indeed, the presence of drainage infrastructures and impervious surfaces was positively associated with concentrations of dissolved organic
carbon (DOC), soluble reactive phosphorus (SRP), total phosphorus (TP), ammonium, nitrates and nitrites, and electrical conductivity in urban streams26,27,28. Some of these compounds can be
used as nutrients by aquatic bacteria8,29. Thus, heavily impacted urban water bodies with high availability of these nutrients and other suitable environmental conditions (e.g., relatively
high temperatures and a wide range of colonizable niches) may become hot habitats for _E. coli_ strains showing long-term persistence30,31,32,33. Previous studies have suggested that
environmental persistence of _E. coli_ in urban freshwater systems is influenced by abiotic factors such as soil moisture, temperature and nutrient availability7,34. Furthermore, it was
shown that high levels of soluble nutrients (e.g., DOC and phosphorus) have a positive influence on growth and survival of _E. coli_ in the water column of urban streams8. Moreover, the
input of black and grey waters into streams entails the dispersal of allochthonous bacteria, representing one of the major drivers of shifts in the microbial community composition of these
systems35,36,37. Septic tanks are also known to contribute to non-point pollution through leakage, acting as a source of pathogens, dissolved nutrients and metals to groundwater and nearby
streams38,39. Likewise, sanitary systems such as sewer networks, if present, cause pollution by leakage and subsequent exfiltration to water bodies39,40. Hence, ecological niche conditions
and human-facilitated dispersal processes may also play an important role in modulating _E. coli_ population structure in urban surface waters. In this work we analyzed the ecological
processes affecting the spatial structure of _E. coli_ in a heavily polluted urban stream network of Buenos Aires province, Argentina. The watershed was characterized as a social-ecological
and technological system in order to analyze the contributions of local urban infrastructure and environmental conditions. Spatial processes structuring _E. coli_ populations that were not
explained by the latter were assessed by including asymmetric eigenvector maps. We hypothesize that the density of input sources to streams and the features of the ecological habitat
determine the spatial patterns of abundance and phylogenetic composition of _E. coli_. In this context, we predict that (1) _E. coli_ abundance will be positively associated with the density
of different urban input sources and higher nutrient availability, (2) that the occurrence of phylogroup B1 will be associated with higher nutrient availability and aquatic vegetation
coverage and (3) that the occurrence of low-frequency _E. coli_ phylogroups (D and F) will be positively associated with locations of higher urbanization. Finally, we discuss our results in
relation to the different ecological processes that could influence the dynamics of _E. coli_ and their implications for urban water management. METHODS AND MATERIALS STUDY AREA AND SAMPLING
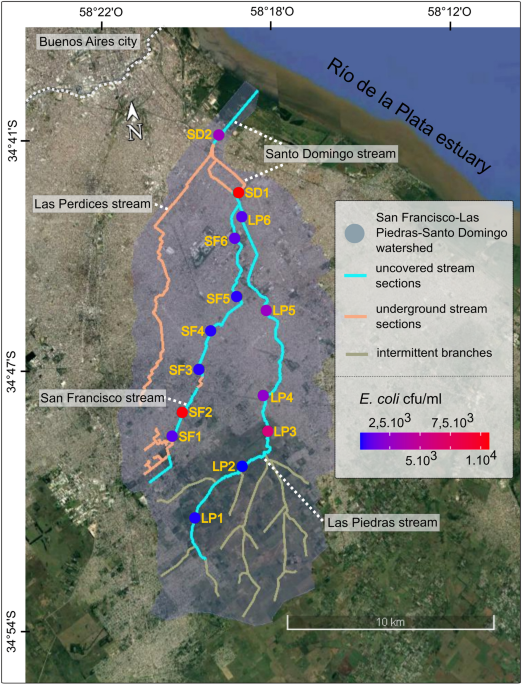
PROCEDURE The watershed of San Francisco, Las Piedras and Santo Domingo streams is located in the Pampean plain, in the south of the highly urbanized Buenos Aires Metropolitan Area (AMBA),
surrounding Buenos Aires city (Fig. 1). This watershed encompasses a total surface area of approximately 160 km2, with a longitudinal extension of about 23 km. The streams draining this area
are affected by multiple anthropogenic stressors41. The watershed and stream network have been highly modified by rectifications, channel incisions and some streams were partially piped;
one of the latter (Las Perdices stream) was even relocated into this watershed. In addition, urban growth in the AMBA was not accompanied by investments in sanitary infrastructure and as a
result, local water bodies receive the impact of untreated sewage and domestic effluents42,43,44. We selected 14 sampling sites across the watershed representing a broad range of urban
characteristics related to population density and coverage of sanitary services. Data were obtained from the Census Data Center45. The sampling campaign was conducted in summer, the season
with the most favorable mean temperature for _E. coli_ survival, during 4 consecutive days in February 2018. A dry period of at least 4 days was considered before the sampling camping and no
rainfall were recorded during its duration. Sites across the stream network were visited randomly at morning-noon to avoid spatio-temporal dependence. Habitat heterogeneity was assessed
along a 50 m-longitudinal transect established along the main watercourse at each sampling site, where sub-samples were taken at 0 m, 25 m and 50 m. On each sub-sampling site, pH, dissolved
oxygen (DO), conductivity and temperature (T) were measured in situ from the sub-surface layer of the water column, using a Lutron WA-2017SD multiparameter field sensor. Local hydraulic and
habitat parameters of the sampled stream section were assessed in terms of physical dimensions (width and depth), flow velocity and macrophyte coverage. Flow velocity was determined using
the flotation method by triplicate and water flow from physical and flow velocity parameters46. Mean values of flow velocity, depth and water flow were calculated per site. Macrophyte
coverage was estimated according to the Braun-Blanquet methodology, by establishing a 0.5 m width plot perpendicular to the flow and accounting for emerged and floating-leaved macrophytes47.
Water samples from each sub-sampling site were collected in clean 250-ml bottles for the analysis of dissolved nutrients and other analytes and in sterile 810-ml plastic bags (Hixwer) for
measuring microbiological parameters. Water samples were taken from the sub-surface layer and kept cold until processing in the laboratory. PHYSICOCHEMICAL CHARACTERIZATION Upon arrival at
the laboratory, turbidity was measured with a turbidimeter HACH 2100P. Samples were filtered simultaneously for further quantification of soluble analytes. First, samples were filtered
through a cellulose nitrate filter of 0.47 µm-pore diameter (Sartorius) for subsequent analysis of soluble reactive phosphorus (SRP), ammonium and nitrates. Then, they were passed through a
polycarbonate filter of 0.2 µm-pore diameter (Merck Millipore) for dissolved organic carbon (DOC), soluble iron and chloride. SRP was determined by the molybdovanadate method, ammonium by
the salicylate method and nitrate plus nitrite by the cadmium reduction method48. Measurements were made by colorimetric analysis with a spectrophotometer Hach® DR 2800 within 48 h of sample
collection; limits of quantification (LOQ) were 0.1 mg/L for SRP and nitrates and 0.4 mg/L for ammonium. Ammonia and nitrate plus nitrite were considered together as dissolved inorganic
nitrogen (DIN). For DOC determination, filtered samples were acidified with high-quality sulfuric acid (Merck) and measurements were performed with the high-temperature combustion method
using a total organic carbon analyzer Shimadzu 5000A; LOQ was 0.1 mg/L48. Chlorides were measured by the iodometric titration method; LOQ was 4 mg/L48. Finally, filtered samples pre-treated
with ultrapure nitric acid (Merck) were analyzed for soluble iron by inductively coupled plasma-mass spectrometry (ICP-MS) using a mass spectrometer Agilent 7500cx; LOQ was 0.3 µg/L.
_ESCHERICHIA COLI_ ABUNDANCE AND PHYLOGENETIC GROUP ANNOTATION _Escherichia coli_ abundance was determined for each sample (42 samples in total, three per site) by plate counting using
Chromocult® Coliform Agar selective medium (MilliporeSigma). Serial dilutions in sterile ultrapure water were performed on each sample in triplicate and plates were cultured for 24 h at 37
°C. After scoring for blue- to purple-colored colonies in the best dilution for each sample, triplicates counts were averaged per sample and relativized to one milliliter of volume. The
detection limit was 200 cfu/100 mL. To obtain the relative phylogenetic composition of _E. coli_ per location, in a first step, blue- to purple-colored colonies from the Chromocult® plates
from each site were streaked onto Levine E.M.B. (Eosin Methylene Blue) agar plates and cultured for 24 h at 36 °C. The streaking procedure was repeated at least once under the same culture
conditions to ensure the purity of each isolate. Isolates yielding typical responses for _E. coli_ on all media were designated _E. coli_, while those exhibiting no typical phenotypic
responses in any of the media were further screened using a colony PCR method with primers directed to _E. coli_ essential _trp_A gene49. A total of 327 isolates were reliably assigned to
_E. coli_, ranging from 18 to 33 isolates per sampled site. Isolates were randomly selected from the sub-sampling plates (see Table S1, Supplementary Appendix A1 for total counts per site).
Phylogenetic annotation within the _E. coli_ intraspecific groups was then performed using the Clermont’s multiplex PCR method13. The closely related phylogroups G and F were grouped
together because this strategy is unable to differentiate one from the other12. Selected isolates were grown overnight in MacConkey broth at 37 °C with continuous shaking and used as
templates in the PCR assays. The first round of phylogroup assignment was performed through the amplification of the _ara_A, _chu_A, _yja_A and TspE4.C2 genes. A remarkable advantage of this
method is that at least one of the targeted genes is guaranteed to be amplified, thus allowing a positive control of species identity. Additional primers were used to identify phylogroups E
and C if needed, by detection of _arp_A and _trp_A genes, respectively. A double PCR method, based on _aes_ and _chu_A allele‐specific amplification, was employed to assign an _Escherichia_
strain a cryptic lineage membership50,51. All PCR reactions were carried out in a 20 µL-total volume containing 2 µL of templates and 10 µL of 2X GoTaq® Green Master Mix (Promega) following
the published literature13,51,52. The complete assignment of isolates to phylogroups per site is shown in Table S1 (Supplementary Appendix A1). DEMOGRAPHIC, HYDRAULIC AND SANITARY
INFRASTRUCTURE PARAMETERS A 500-m radius circular area was established around each site to survey the total number of dwellings and residents and the total number of dwellings with sanitary
sewers, septic tanks and potable water, using open data provided by national agencies45. The impervious surface coverage was calculated through GIS image processing (QGIS v. 2.18.20) coupled
with the Semi-automatic Classification Plugin algorithm, employing Landsat 8 satellite images of the watershed53. This methodology is based on the preliminary creation of a spectral firm by
a supervised classification procedure for the generation of regions of interest (ROIs). Each pixel in the ROI was categorized as impervious or pervious area. The trained ROIs were employed
with the Spectral Angle Mapper algorithm to characterize the satellite image of the watershed. Impervious surface coverage was first calculated by manual digitalization of about 30 ha
distributed homogeneously throughout the basin, obtained from a Google Maps image 54,55,. This was used to generate polygons representing areas with different percentages of imperviousness.
Permeable areas showed a limit of impermeability of 5%. Finally, considering a 500-m radius buffer area for each sampling site, GIS processing tools were used to calculate the areas for the
two permeability types, to further express the impervious surface as a percentage within each buffer area. To estimate drainage and road densities, layouts of the pluvial and road networks
were provided by local and provincial administrations agencies. Data were intersected with the buffer areas to calculate the length of their respective pluvial and road sections. ESTIMATION
OF SPATIAL PREDICTORS Asymmetric Eigenvector Maps (AEMs) were generated to model directional spatial processes underlying streams network singularities54,55,56. These AEMs were based on the
geographical distribution of the sampling sites, and the connectivity matrix among sites was constructed considering the upstream–downstream flux and a maximum spatial neighborhood distance
of 5 km between sites. The AEMs also included weight vectors obtained from the connectivity matrix, which determined the connection strength between samples according to their geographic
distance. A two-sided Moran’s test (999 permutations; _p_ < 0.05) was used to retain AEMs showing significant positive or negative spatial autocorrelations (see Supplementary Fig. S1 for
more details)57. Finally, _forward selection_ procedures were carried out separately for each response variable to retain AEMs significantly associated with them. This yielded 6 and 11 AEMs
that were significantly associated with _E. coli_ abundance and phylogenetic composition, respectively. The four AEMs gathering the greatest R2 contribution were employed to avoid
multicollinearity in further analysis involving _E. coli_ phylogenetic composition. These procedures were conducted using the packages _adespatial_ (v 0.3–8), _ade4_ (v 1.7–15), _sp_ (v
1.3–2) and _spdep_ (v 1.1–3) in R. For more details on the methodology for spatial-predictor selection, see Appendix A2 of the Supplementary Material. DATA ANALYSIS We first calculated the
phylogroup specific diversity and dominance at each site for an initial characterization of the phylogroup population structure58,59. Phylogroup diversity was obtained by counting the number
of different phylogroups present at each site, while Simpson’s dominance index (D) was calculated as the weighted arithmetic mean of their proportional abundances58. The association between
phylogroup diversity, dominance and _E. coli_ mean abundance was analyzed using a Pearson’s correlation analysis. Different statistical approaches were applied to analyze relationships
among predictors. Initially, two sets of predictor variables were established: an environmental matrix including local physicochemical and habitat conditions and an urban infrastructure set,
combining demographic information with different aspects of sanitary infrastructure at the watershed scale. A Principal Component Analysis (PCA) was performed to analyze the association
between sites and environmental and urban infrastructure variables60. Additionally, a Pearson’s correlation matrix with hierarchical clustering was performed among variables. The
significance of the correlation coefficients (Pearson’s ρ) was evaluated with the paired-samples correlation test adjusted for multiple comparisons (Holm’s method)61. In addition, we
analyzed the network of global correlations between pairs of variables showing an absolute Pearson’s ρ greater than 0.5. To understand the importance of each parameter in determining the
whole network’s structure, we estimated associated centrality measures such as Expected Influence (EI), which represents the strength of a node’s influence within the network62. A variation
partitioning analysis was performed to disentangle the pure and shared effects of the local environment, urban infrastructure and spatial predictors determining the patterns of abundance and
phylogroup composition (based on presence-absence data) of _E. coli_63. An adjusted multivariate redundancy statistic (Ra2) was used to analyze the proportion of variance explained by each
component. To reduce the number of variables and to avoid multicollinearity between predictors, a set of multivariate analyses were performed. This was accomplished by identifying several
groups of covariate variables by means of a PCA analysis applied to the environmental and infrastructure matrices separately, along with the use of variance inflation factors (for more
details on the procedure for variable selection, see Appendix A3 of the Supplementary Material). For variance partitioning and partial redundancy analyzes, the _E. coli_ abundance and the
predictor matrices were previously standardized by the _Standardization_ method64. In accordance with Leps & Smilauer65, we preliminarily assessed the use of a linear constrained model
(RDA) onto phylogroup composition data, by considering the length of the gradient obtained with a Detrended Correspondence Analysis (DCA; 1st axis length = 1.44). The significance of global
models and variables was further evaluated through partial redundancy analysis (pRDA), which was subjected to different conditioning constraints. In the case of the _E. coli_ abundance, two
different constraints were implemented (1) a pRDA analysis of the environmental or infrastructure matrices, conditioned by each other, and (2) a pRDA analysis of spatial predictors
conditioned by the rest of the predictor sets. In contrast, for the occurrence of _E. coli_ phylogroups, each predictor matrix was conditioned by the rest of the variables. In all cases,
statistical significance of pRDA was tested using a restricted Monte Carlo Permutation Test of the residuals of the full model (9999 permutations), to account for our nested sampling design
and the multilevel structure of the explanatory variables66. Both normality and lack of structure in residuals were tested. Statistical procedures were implemented with the packages _vegan_
(version 2.5–6), _corrplot_ (version 0.84), _qgraph_ (version 1.6.5) and _permute_ (version 0.9–5) in R. RESULTS PATTERNS OF _E. COLI_ ABUNDANCE ASSOCIATED WITH ENVIRONMENTAL AND URBAN
INFRASTRUCTURE FEATURES To gain insight into the ecological processes modulating the population structure of _E. coli_, we assessed the joint and independent contributions of urban features
and local stream habitat conditions across 14 stream reaches (locations) distributed throughout a heavily polluted urban stream network with heterogeneous built-in sanitation and drainage
infrastructure (Fig. 1). The characterization of urban features included demographic, hydraulic (impervious surfaces, drainage density and road density) and sanitary conditions (sanitary
sewers, septic tanks and potable water) (Table 1). Local habitats were characterized for their physicochemical profile (pH, conductivity, temperature, dissolved oxygen, DIN, SRP, DOC,
turbidity, chlorides and iron) and for local hydraulic and habitat parameters of the stream section (physical dimensions, water flow, flow velocity and macrophyte coverage) (Table 1).
Spatial predictors were also included in the analysis to account for directional and correlated effects (see Appendix A2 of the Supplementary Material). _E. coli_ abundance was quantified at
each location and the phylogenetic affiliation of isolates was assigned by molecular methods. The locations surveyed had a wide variability in environmental and urban infrastructure across
the hydrological network (Table 1 and Fig. 1). _E. coli_ abundance exceeded the level recommended by the US-EPA 20121 of 235 cfu/100 mL for recreational freshwaters at all studied sites,
which poses a great risk for people in direct contact with the studied streams (see Table S1, Appendix A1 of the Supplementary Material for detailed results). The analysis of urbanization
proxies (infrastructure coverage, impervious surface coverage and population density) allowed to characterize the watershed as predominantly urban (mean impervious surface of 53%), with a
low-urbanized area located in the headwater of Las Piedras stream. The rest of the basin is highly urbanized and values of impervious surface are higher downstream (> 70%). However, the
coverage of sanitary infrastructure services, such as drinking water or sewerage, is heterogeneous across the watershed, reflecting differences in the level and quality of urbanization. A
PCA revealed a gradient of urban infrastructure along the watershed (Fig. 2a) and grouped locations into distinct clusters based on their features. A first cluster includes locations from
the upper section of Las Piedras stream, with higher macrophyte coverage and DIN, together with lower infrastructure coverage. A second cluster groups locations from the upper section of the
San Francisco stream, which mainly show intermediate infrastructure coverage. Lastly, a third cluster is composed of locations from densely populated areas with a high development of
hydraulic and sanitary infrastructure, which are associated with lower levels of nutrients and high flow velocity. One location in the Santo Domingo stream (SD1) was not grouped in any of
the clusters identified, mainly associated with elevated levels of DOC, _E. coli_ abundance and turbidity. A network of global Pearson’s correlations was used to explore co-variation
patterns between variables (see Figure S4, Supplementary Appendix A4 for the overall significance analysis). The obtained network was analyzed in terms of the influence or relative
importance of each variable (see Fig. 2b and Table S4 in Appendix A4 of the Supplementary Material for full-network metrics). Urban infrastructure variables were ordered close to each other
by their positive and significant correlations, mainly interacting through the proportion of impervious surface (expected influence, |EI|= 4.80), drinking water coverage (|EI|= 3.65) and
population density (|EI|= 3.45). Among environmental variables, flow velocity (|EI|= 2.48), water flow (|EI|= 2.46), and with lower relative influence, macrophyte coverage (|EI|= 1.91)
concentrated the links with urban infrastructure, mainly interacting with sanitary sewer density (|EI|= 2.95) and impervious surface. A second branch of interactions was evidenced between
impervious surface and conductivity (|EI|= 2.54) and chlorides (|EI|= 1.52). Moreover, pH (|EI|= 4.82) and DOC (|EI|= 3.60) represented key variables within the environmental matrix,
gathering the largest number of links. Local environmental variables such as DOC, SRP, Iron, conductivity, chlorides and DIN were also clustered in a second positive and significant
co-variation group. _E. coli_ abundance was significantly and positively associated with nutrients such as SRP and DOC, and with physicochemical conditions such as pH (−) and conductivity
(+), suggesting that urbanization parameters may indirectly influence _E. coli_ abundance through environmental factors. Finally, a cluster of strong positive and negative significant
correlations was found among sanitary sewer density, pH, water flow, turbidity, depth and macrophyte coverage. SPATIAL DISTRIBUTION OF _E. COLI_ PHYLOGENETIC GROUPS A total of 327
environmental isolates were characterized based on the Clermont's multiplex PCR method for _E. coli_ phylogroup annotation (see Table S1, Supplementary Appendix A1 for the absolute
abundances detected per site). We detected most of the phylogenetic groups, except for phylogroup C. Phylogroup A was the most abundant in all locations sampled, with a relative frequency of
up to 50% at most sites, followed by phylogroup B1 (Fig. 3). Mean relative frequency was 64% for phylogroup A, 16% for B1, 10% for D, 5% for F/G, 3% for E and 2% for B2 (Fig. 3b). Notably,
a single isolate of the _cryptic clade_ IV (further confirmed by a specific multiplex PCR assay for the identification of cryptic clades within the genus _Escherichia_) was collected in
location SF5. To our knowledge, this is the first report of a cryptic clade member in surface waters of South America. A correlation analysis of community structure metrics applied to _E.
coli_ phylogenetic composition showed that phylogroup diversity was negatively correlated with total mean abundance of _E. coli_ (coefficient of correlation ρ = − 0.56; _p_ = 0.05) (Fig.
3c). In addition, Simpson’s dominance index (D) was negatively correlated with phylogroup diversity (coefficient of correlation ρ − 0.87; _p_ = 1.10−4). DISENTANGLING THE EFFECTS OF
ENVIRONMENTAL, URBAN AND SPATIAL FACTORS ON PATTERNS OF _E. COLI_ ABUNDANCE AND PHYLOGENETIC COMPOSITION A variance partition analysis (Fig. 4) showed that important independent effects of
environmental and urban infrastructure predictors (23% and 18% of the fraction shared with the spatial matrix, respectively) affect the distribution of _E. coli_ abundance. At the same time,
the three predictor sets together explained 13% of the total variance. The spatial matrix of AEMs showed a strong pure contribution (27%), while environmental and infrastructure matrices
exhibited remarkably lower pure contributions (< 3%) (Fig. 4a). In terms of overall influence, 42% of total contribution to the explained variance was related to the environmental matrix
and 39% to the urban infrastructure matrix, indicating a similar contribution of both sets of predictors to the observed patterns of _E. coli_ abundance. The high contribution of spatial
factors in shared and pure fractions indicates that _E. coli_ abundance has a strong directional spatial structure, potentially reflecting distinct spatially structured processes (i.e.,
different hydrological, environmental and infrastructure patterns). To further assess the contribution of spatially structured environmental and urban infrastructure variables, a pRDA was
performed, where each explanatory set was conditioned by the other (Fig. 4a). Results showed that both environmental (F7,31 = 4.48, _P_ < 0.05) and urban (F3,31 = 7.38, _P_ < 0.05)
matrices were significant in explaining _E. coli_ abundance. Within the environmental set, significant variables were macrophyte coverage (score = 0.43; F1,31 = 12.38, _P_ < 0.05) and DIN
(score = 0.43; F1,31 = 12.81, _P_ < 0.05). In regard to the urban infrastructure matrix, sanitary sewer density had a significant contribution (score = 0.51; F1,31 = 18.02, _P_ <
0.05). As well, several coarse- and fine-grade AEMs of the spatial matrix had significant pure contributions (Fig. 4a; see more details in Appendix A2 of the Supplementary Material, Table
S2). In contrast to the results obtained for abundance, spatial variability in phylogroup composition at the watershed (based on phylogroup presence-absence data) was mostly explained by
pure contributions of the three matrices (Fig. 4b). The contribution of spatial factors was the largest (46%), followed by environmental (31%) and urban infrastructure factors (9%). Partial
RDA of environmental variables, controlling for the effect of urban infrastructure and spatial AEMs, was statistically significant (F7,24 = 27.69, _P_ < 0.05). The following variables
were significant: DIN (F1,24 = 25.95, _P_ < 0.05), turbidity (F1,24 = 22.99, _P_ < 0.05), SRP (F1,24 = 20.41, _P_ < 0.05), depth (F1,24 = 9.03, _P_ < 0.05), flow velocity (F1,24
= 13.33, _P_ < 0.05) and macrophytes coverage (F1,24 = 7.04, _P_ < 0.05) (Fig. 4b). The first two pRDA axes were also found to be significant: RDA1 accounted for an explained
proportion of 42% (F1,26 = 98.55, _P_ < 0.05) and RDA2 of 31% (F1,26 = 72.29, _P_ < 0.05). RDA1 was mainly positively related to macrophyte coverage (score = 0.29), flow velocity
(score = 0.25) and depth (score = 0.13), and negatively related to turbidity (score = − 0.29) and potential nutrients DIN (score = − 0.13) and SRP (score = − 0.07) (Fig. 5a), while RDA2
was positively related to DIN (score = 0.20) and turbidity (score = 0.19), and negatively associated to depth (score = − 0.28), SRP (score = − 0.12) and macrophyte coverage (score = −
0.10). Phylogroups D, E, F/G and B2 were strongly and negatively correlated with RDA1 suggesting their association with turbidity and higher nutrients conditions, while B1 was slightly and
positively associated with it, thus distinguishing between dominant and less frequent phylogroups. In addition, partial RDA of urban infrastructure predictors, controlling for the effect of
environmental and spatial variables was statistically significant (F3,24 = 17.39, _P_ < 0.05), with a significant association of phylogroup composition with drainage density (F1,24 =
11.49, _P_ < 0.05), septic tanks density (F1,24 = 9.12, _P_ < 0.05) and sanitary sewers density (F1,24 = 7.27, _P_ < 0.05) (Fig. 4b). Significant axis RDA1 (F1,24 = 25.53, _P_ <
0.05), with an explained proportion of 34%, was negatively related to septic tank density (score = − 0.29) and drainage density (score = − 0.24). Moreover, phylogroups B2, E and, to a
lesser extent, B1 were negatively associated with RDA1, while F/G and D were positively associated with this axis (Fig. 5b). Axis RDA2, which accounted for 28% of the explained variance, was
also significant (F1,24 = 21.10, _P_ < 0.05) and positively related to the drainage and sanitary sewers densities (scores 0.33 and 0.31, respectively), while septic tanks density was
negatively associated to this axis (score = − 0.21). Phylogroups F/G and E, and cryptic clade IV and negatively related to RDA2, in contrast to phylogroups B2, D and B1. Finally, partial
RDA of spatial factors, constrained by environmental and urban infrastructure, showed that all the included AEMs (ranging from broad- to fine-scale resolution levels) were significant in
explaining spatial variability (see Table S2 and Figure S2 in Appendix A2 of the Supplementary Material for more information). DISCUSSION Our study indicates that the spatial structuring of
_E. coli_ in a highly polluted urban stream network with favorable growth conditions is strongly influenced by the hydraulic and sanitary infrastructure and local ecological features of the
habitat. The spatial distribution of _E. coli_ abundance and the phylogenetic composition of its population reflect the complex interactions between the socio-technological attributes of the
urban environment and the ecological conditions of the stream habitat, supporting our original hypothesis. We found that both urban infrastructure and micro-habitat conditions, which were
in a straight relationship with spatial predictors, acted as important drivers of _E. coli_ abundance in the streams network studied. Moreover, _E. coli_ abundance was negatively correlated
with phylogroup diversity. On the other hand, _E. coli_ phylogenetic composition was driven by independent effects of spatial, infrastructure and environmental variables, as well as by a
strong negative relationship between phylogroup diversity and dominance. We hypothesize that these results reflect the influence of distinct ecological processes on _E. coli_ populations,
thereby opening future avenues of research in other urban watersheds and additional hydro-climatic conditions. It has been shown that the effects of geomorphological modifications made to
urban streams (channel incisions, rectifications, concrete riverbanks, buried sections) result in a process of ecological simplification and in the predominance of exogenous control over
internal equilibrium states, leading to undesirable resilient states67,68. In this vein, we hypothesize that the indivisible effect of infrastructure, environmental and spatial predictors
explaining _E. coli_ abundance patterns reflects the effect of large watershed-scale processes that cause a significant modification to local habitats, modulating microhabitat features that
influence _E. coli_ availability. Our hypothesis is supported by the negative co-variation observed between urbanization proxies (e.g., drainage density) and microhabitat features (e.g.,
macrophyte coverage). Regarding the independent effects of environmental and urban conditions on _E. coli_ abundance, we found a positive link with sanitary sewer density, which is in line
with previous studies reporting the contribution of sewer leaks68,69. Furthermore, the association of _E. coli_ abundance with macrophyte coverage suggests that its growth may be favored in
locations with lower urbanization levels and aquatic vegetation. Indeed, macrophytes are known to be colonizable surfaces for _E. coli_32. Once established, and if nutrient levels are
sufficient, bacteria may grow and eventually release cells into freshwater bodies. Surbeck et al.8 reported that _E. coli_ grows above threshold concentrations of DOC (7 mg/L) and SRP (0.07
mg/L), evidencing its ability to exploit soluble nutrients available in urban freshwaters. The nutrient concentrations detected across the basin were above these values, thus providing
favorable environmental conditions for growth. In addition, we found a significant correlation between DOC and the presence of _E. coli_, while other potential nutrients (DIN and Iron) were
also spatially correlated with DOC. Altogether, these results validate our first prediction of a positive association of _E. coli_ abundance with urban income sources and high nutrient
availability. We found several remarkable aspects in relation to phylogenetic composition. Phylogroups A and B1 showed a co-dominant structure throughout the sampled sites, suggesting their
wide ubiquity regardless of differences in environmental conditions. This is in agreement with Petit et al.16 who reported co-dominance of A and B1 in the water column, with an increase in
the frequencies of phylogroups A, D and F in urban locations. With respect to human gut microbiomes in South America, Stoppe69 found a predominance of phylogroup A, followed by B2, B1 and D,
in an urban population from Brazil, in line with the results of Escobar-Páramo et al.70. On this basis, the dominance of the phylogroups detected in the stream network studied here suggests
that human fecal contamination is widespread throughout the watershed. In our study, lower frequency phylogroups (D, E, F/G and B2) were associated with water turbidity, which is positively
correlated with water flow and the local density of sanitary sewers around each sampled site, and higher nutrient availability (e.g., SRP and DIN), an association that can be interpreted as
a product of common sources of contamination. Our results also suggest that the occurrence of lower frequency phylogroups (B2, D, E, F/G) are mostly associated with recent external
contributions from urban drainage infrastructures or septic tank exfiltration. This is consistent with the idea that some of them (such as D or F) occur in highly urbanized locations. On the
other hand, B1 was linked to contrasting conditions where aquatic vegetation prevails, which is partially consistent with our second prediction, while there were no strong associations to
urban infrastructures. Bearing in mind that B1 can be naturalized in water bodies -thus forming part of the indigenous microbiota- and that this phylogroup is able to colonize surfaces like
macrophytes through biofilm development, our results may be interpreted as a positive environmental effect on B1 growth18,19,71,72. Finally, one strain belonging to the cryptic clade IV was
isolated from a highly populated and urbanized location with low levels of sewerage infrastructure. Altogether, our results can be interpreted in terms of different scenarios behind _E.
coli_ abundance and its phylogenetic composition in urban streams with favorable growth conditions (Fig. 6). A first scenario corresponds to locations with a relatively low level of
disturbance, characterized by low infrastructure and stable habitat conditions, including the presence of high macrophyte coverage. These conditions, together with a high availability of
nutrients, would promote high rates of bacterial growth and competitive exclusion of ecologically similar phylogroups. In this context, _E. coli_ strains with the potential for survival and
growth dominate the population structure, thus reducing the survival probability of lower frequency phylogroups. The second scenario involves highly disturbed locations with heavy external
inputs. The lack of aquatic vegetation in local habitats and the periodic disturbances produced by drainage networks, may limit bacterial growth and offset competitive exclusion. The
phylogenetic composition of _E. coli_ populations in these habitats may be influenced by dispersal, facilitated through urban infrastructures and other diffuse sources like septic tanks
leakage. Finally, the sanitary sewer network was also observed to positively contribute to the _E. coli_ abundance, exerting its influence on _E. coli_ occurrence irrespective of the
environmental disturbance conditions. Finally, we want to comment on some methodological issues of our analysis. First, we uncovered that the spatial predictors were relevant for explaining
_E. coli_ abundance and phylogenetic composition (Fig. 4). The selected broad- to fine-scale asymmetric eigenvectors reflect an expectable directional process through the stream network,
involving a strong level of autocorrelation between close locations. They also reveal distinct spatially structured processes underlying transport-based (hydrological), spatial environmental
or infrastructure patterns. It is worthy to note that the lack of updated information on public sanitary conditions and demographic characteristics may have introduced some bias into the
selected AEMs showing pure spatial contributions. Therefore, the usage of spatial predictors allowed us to pull out spatial autocorrelation processes inherent to the structure of stream
networks, enabling a more accurate analysis of the influence of local habitats or urban infrastructure features throughout the watershed. Second, the results and hypothetical mechanisms
analyzed in this work must be assumed as general trends of the drivers for phylogroup diversity, since they arise from a single point in time, in favorable growth conditions (summer season).
The use of presence/absence data due to limitations in sample size also implies a simplification of the real population structure, thus limiting further inferences. The analysis of the
spatio-temporal dynamics of _E. coli_ in urban several watersheds and the collection of a higher number of _E. coli_ isolates will allow us to reach more extensive conclusions, while the
present work offers contrastable hypothesis for further evaluation. To conclude, our results provide information useful for global comparisons of _E. coli_ persistence in the environment,
while also having important implications for water quality assessment, as they question the use of _E. coli_ as a proxy for recent microbiological pollution. In this regard, the genomic
analysis of isolates, together with experimental assays may provide useful information on the determinants of _E. coli_ environmental persistence and the differential fitness of phylogroups.
REFERENCES * _2012 Recreational Water Quality Criteria_. (U. S. Environmental Protection Agency, 2012). * Lee, C. M. _et al._ Persistence of fecal indicator bacteria in Santa Monica Bay
beach sediments. _Water Res._ 40, 2593–2602 (2006). Article CAS PubMed Google Scholar * Luo, C. _et al._ Genome sequencing of environmental _Escherichia coli_ expands understanding of
the ecology and speciation of the model bacterial species. _Proc. Natl. Acad. Sci._ 108, 7200–7205 (2011). Article ADS CAS PubMed PubMed Central Google Scholar * Ishii, S., Ksoll, W.
B., Hicks, R. E. & Sadowsky, M. J. Presence and growth of naturalized _Escherichia coli_ in temperate soils from lake superior watersheds. _Appl. Environ. Microbiol._ 72, 612–621 (2006).
Article ADS CAS PubMed PubMed Central Google Scholar * Rochelle-Newall, E., Nguyen, T. M. H., Le, T. P. Q., Sengtaheuanghoung, O. & Ribolzi, O. A short review of fecal indicator
bacteria in tropical aquatic ecosystems: Knowledge gaps and future directions. _Front. Microbiol._ 6, 1–15 (2015). Article Google Scholar * Tymensen, L. D. _et al._ Comparative accessory
gene fingerprinting of surface water _Escherichia coli_ reveals genetically diverse naturalized population. _J. Appl. Microbiol._ 119, 263–277 (2015). Article CAS PubMed Google Scholar *
Ishii, S. & Sadowsky, M. J. _Escherichia coli_ in the environment: Implications for water quality and human health. _Microbes and environments / JSME_ 23, 101–108 (2008). Article
Google Scholar * Surbeck, C. Q., Jiang, S. C. & Grant, S. B. Ecological control of fecal indicator bacteria in an urban stream. _Environ. Sci. Technol._ 44, 631–637 (2010). Article ADS
CAS PubMed Google Scholar * Jang, J. _et al._ Environmental _Escherichia coli_: Ecology and public health implications—A review. _J. Appl. Microbiol._ 123(3), 570–581.
https://doi.org/10.1111/jam.13468 (2017). Article CAS PubMed Google Scholar * Van Elsas, J. D., Semenov, A. V., Costa, R. & Trevors, J. T. Survival of _Escherichia coli_ in the
environment: Fundamental and public health aspects. _ISME J._ 5, 173–183 (2010). Article PubMed PubMed Central Google Scholar * Jaureguy, F. _et al._ Phylogenetic and genomic diversity
of human bacteremic _Escherichia coli_ strains. _BMC Genomics_ 9, 560 (2008). Article PubMed PubMed Central CAS Google Scholar * Clermont, O. _et al._ Characterization and rapid
identification of phylogroup G in _Escherichia coli_, a lineage with high virulence and antibiotic resistance potential. _Environ. Microbiol._ 21, 3107–3117 (2019). Article CAS PubMed
Google Scholar * Clermont, O., Christenson, J. K., Denamur, E. & Gordon, D. M. The Clermont _Escherichia coli_ phylo-typing method revisited: Improvement of specificity and detection of
new phylo-groups. _Environ. Microbiol. Rep._ https://doi.org/10.1111/1758-2229.12019 (2012). Article PubMed Google Scholar * Ratajczak, M. _et al._ Influence of hydrological conditions
on the _Escherichia coli_ population structure in the water of a creek on a rural watershed. _BMC Microbiol._ 10, 1–10 (2010). Article CAS Google Scholar * Johnson, J. R. _et al._
Phylogenetic backgrounds and virulence associated traits of _Escherichia coli_ isolates from surface waters and diverse animals in Minnesota and Wisconsin. _Appl. Environ. Microbiol._ 83,
1–33 (2017). Article CAS Google Scholar * Petit, F. _et al._ Change in the structure of _Escherichia coli_ population and the pattern of virulence genes along a rural aquatic continuum.
_Front. Microbiol._ 8, 1–14 (2017). Article Google Scholar * Kraft, N. J. B. _et al._ Community assembly, coexistence and the environmental filtering metaphor. _Funct. Ecol._ 29, 592–599
(2015). Article Google Scholar * Berthe, T., Ratajczak, M., Clermont, O., Denamur, E. & Petit, F. Evidence for coexistence of distinct _Escherichia coli_ populations in various aquatic
environments and their survival in estuary water. _Appl. Environ. Microbiol._ 79, 4684–4693 (2013). Article ADS CAS PubMed PubMed Central Google Scholar * Méric, G., Kemsley, E. K.,
Falush, D., Saggers, E. J. & Lucchini, S. Phylogenetic distribution of traits associated with plant colonization in _Escherichia coli_. _Environ. Microbiol._ 15, 487–501 (2013). Article
PubMed CAS Google Scholar * Walk, S. T. The “Cryptic” _Escherichia_. _EcoSal Plus_ 6, 2 (2015). * Ingle, D. J. _et al._ Biofilm formation by and thermal niche and virulence
characteristics of _Escherichia_ spp. _Appl. Environ. Microbiol._ 77, 2695–2700 (2011). Article ADS CAS PubMed PubMed Central Google Scholar * Elmqvist, T. _The Urban Planet: Knowledge
Towards Sustainable Cities_ (Cambridge University Press, 2018). Book Google Scholar * Hosen, J. D., Febria, C. M., Crump, B. C. & Palmer, M. A. Watershed urbanization linked to
differences in stream bacterial community composition. _Front. Microbiol._ 8, 1–17 (2017). Article Google Scholar * Wang, S.-Y., Sudduth, E. B., Wallenstein, M. D., Wright, J. P. &
Bernhardt, E. S. Watershed urbanization alters the composition and function of stream bacterial communities. _PLoS ONE_ 6, e22972 (2011). Article ADS CAS PubMed PubMed Central Google
Scholar * Bernhardt, E. S., Band, L. E., Walsh, C. J. & Berke, P. E. Understanding, managing, and minimizing urban impacts on surface water nitrogen loading. _Ann. N. Y. Acad. Sci._
1134, 61–96 (2008). Article ADS CAS PubMed Google Scholar * Hosen, J. D., McDonough, O. T., Febria, C. M. & Palmer, M. A. Dissolved organic matter quality and bioavailability
changes across an urbanization gradient in headwater streams. _Environ. Sci. Technol._ 48, 7817–7824 (2014). Article ADS CAS PubMed Google Scholar * Hatt, B. E., Fletcher, T. D., Walsh,
C. J. & Taylor, S. L. The influence of urban density and drainage infrastructure on the concentrations and loads of pollutants in small streams. _Environ. Manage._ 34, 112–124 (2004).
Article PubMed Google Scholar * Smith, R. M., Kaushal, S. S., Beaulieu, J. J., Pennino, M. J. & Welty, C. Influence of infrastructure on water quality and greenhouse gas dynamics in
urban streams. _Biogeosciences_ 14, 2831–2849 (2017). Article ADS CAS Google Scholar * Handler, N. B., Paytan, A., Higgins, C. P., Luthy, R. G. & Boehm, A. B. Human development is
linked to multiple water body impairments along the California coast. _Estuar. Coasts_ 29, 860–870 (2006). Article CAS Google Scholar * Ishii, S. _et al._ Factors controlling long-term
survival and growth of naturalized _Escherichia coli_ populations in temperate field soils. _Microbes Environ._ 25, 8–14 (2010). Article PubMed Google Scholar * Whitman, R. L. _et al._
Microbes in beach sands: Integrating environment, ecology and public health. _Rev. Environ. Sci. Biotechnol._ 13, 329–368 (2014). Article CAS PubMed PubMed Central Google Scholar *
Kleinheinz, G. _et al._ Effect of aquatic macrophytes on the survival of _Escherichia coli_ in a laboratory microcosm. _Lake Reserv. Manage._ 25, 149–154 (2009). Article Google Scholar *
Moreira, S. _et al._ Persistence of _Escherichia coli_ in freshwater periphyton: Biofilm-forming capacity as a selective advantage. _FEMS Microbiol. Ecol._ 79, 608–618 (2012). Article CAS
PubMed Google Scholar * Pachepsky, Y. A. & Shelton, D. R. _Escherichia coli_ and fecal coliforms in freshwater and estuarine sediments. _Crit. Rev. Environ. Sci. Technol._ 41,
1067–1110 (2011). Article CAS Google Scholar * Walsh, C. J. & Kunapo, J. The importance of upland flow paths in determining urban effects on stream ecosystems. _J. N. Am. Benthol.
Soc._ 28, 977–990 (2009). Article Google Scholar * McLellan, S. L., Fisher, J. C. & Newton, R. J. The microbiome of urban waters. _Int. Microbiol._ 18, 141–149 (2015). PubMed PubMed
Central Google Scholar * Newton, R. J. _et al._ Sewage reflects the microbiomes of human populations. _MBio_ 6, 1–9 (2015). Article CAS Google Scholar * Richards, S., Paterson, E.,
Withers, P. J. A. & Stutter, M. Septic tank discharges as multi-pollutant hotspots in catchments. _Sci. Total Environ._ 542, 854–863 (2016). Article ADS CAS PubMed Google Scholar *
Sowah, R. A., Habteselassie, M. Y., Radcliffe, D. E., Bauske, E. & Risse, M. Isolating the impact of septic systems on fecal pollution in streams of suburban watersheds in Georgia,
United States. _Water Res._ 108, 330–338 (2017). Article CAS PubMed Google Scholar * Ly, D. K. & Chui, T. F. M. Modeling sewage leakage to surrounding groundwater and stormwater
drains. _Water Sci. Technol._ 66, 2659–2665 (2012). Article PubMed Google Scholar * Graziano, M., Giorgi, A. & Feijoó, C. Science of the Total Environment Multiple stressors and
social-ecological traps in Pampean streams (Argentina): A conceptual model. _Sci. Total Environ._ 765, 142785 (2020). Article ADS PubMed CAS Google Scholar * Graziano, M. _et al._
Fostering urban transformations in Latin America: Lessons around the ecological management of an urban stream in coproduction with a social movement (Buenos Aires, Argentina). _Ecol. Soc._
24, 13 (2019). Article Google Scholar * Cirelli, A. F. & Ojeda, C. Wastewater management in Greater Buenos Aires, Argentina. _Desalination_ 218, 52–61 (2008). Article CAS Google
Scholar * Elordi, M. L., Lerner, J. E. C. & Porta, A. Evaluación del impacto antrópico sobre la calidad del agua del arroyo Las Piedras, Quilmes, Buenos Aires, Argentina. _Acta
Bioquimica Clinica Latinoamericana_ 50, 669–677 (2016). Google Scholar * _Censo nacional de población, hogares y viviendas 2010 : censo del Bicentenario : resultados definitivos, Serie B nº
2._ (Instituto Nacional de Estadística y Censos, 2012). * Gordon, N. D., McMahon, T. A., Finlayson, B. L., Gippel, C. J. & Nathan, R. J. _Stream Hydrology: An Introduction for
Ecologists_ (Wiley, 2004). Google Scholar * Elosegui, A., Sabater, S. (eds.). _Conceptos y técnicas en ecología fluvial_. 243-251. (Fundación BBVa, 2009) * Baird, R. B., Eaton, A. D., Rice,
E. W., & Bridgewater, L. (eds.)_Standard methods for the examination of water and wastewater, _23. (American Public Health Association, 2017). * Clermont, O., Bonacorsi, S., Bingen, E.
& Bonacorsi, P. Rapid and simple determination of the _Escherichia coli_ phylogenetic group. _Appl. Environ. Microbiol._ 66, 4555–4558 (2000). Article ADS CAS PubMed PubMed Central
Google Scholar * Clermont, O., Gordon, D. M., Brisse, S., Walk, S. T. & Denamur, E. Characterization of the cryptic _Escherichia_ lineages: Rapid identification and prevalence.
_Environ. Microbiol._ 13, 2468–2477 (2011). Article PubMed Google Scholar * Lescat, M. _et al._ Commensal _Escherichia coli_ strains in Guiana reveal a high genetic diversity with
host-dependant population structure. _Environ. Microbiol. Rep._ 5, 9–57 (2013). Article CAS Google Scholar * Clermont, O. _et al._ Evidence for a human-specific _Escherichia coli_ clone.
_Environ. Microbiol._ 10, 1000–1006 (2008). Article CAS PubMed Google Scholar * Congedo, L. Semi-automatic classification plugin for QGIS. _Sapienza Univ_, 1-25 (2013). * Blanchet, F.
G., Legendre, P. & Borcard, D. Forward selection of explanatory variables. _Ecology_ 89, 2623–2632 (2008). Article PubMed Google Scholar * Borcard, D., Gillet, F. & Lengendre, P.
_Numerical Ecology with R_ (Springer, 2018). Book MATH Google Scholar * Legendre, P., Borcard, D. & Roberts, D. W. Variation partitioning involving orthogonal spatial eigenfunction
submodels. _Ecology_ 93, 1234–1240 (2012). Article PubMed Google Scholar * Bivand, R. S. & Wong, D. W. S. Comparing implementations of global and local indicators of spatial
association. _TEST_ 27, 716–748 (2018). Article MathSciNet MATH Google Scholar * Magurran, A. E. _Measuring Biological Diversity_ (Wiley, Hoboken, 2004). Google Scholar * Oksanen, J.
_et al. _Vegan: Ecological Diversity. _R Project_, 368. http://cran.r-project.org (2013) * Wilkinson, L. & Friendly, M. History corner the history of the cluster heat map. _Am. Stat._
63, 179–184 (2009). Article Google Scholar * Wei, T. _et al._ Visualization of a correlation matrix. _Statistician_ 56, 316–324 (2017). Google Scholar * Robinaugh, D. J., Millner, A. J.
& McNally, R. J. Identifying highly influential nodes in the complicated grief network. _J. Abnormal Psychol._ 125(6), 747 (2016). Article Google Scholar * Peres-Neto, P. R., Legendre,
P. L., Dray, S. & Borcard, D. Variation partitioning of species data matrices: Estimation and comparison of fractions. _Ecology_ 87, 2614–2625 (2006). Article PubMed Google Scholar *
Legendre, P. & Gallagher, E. D. Ecologically meaningful transformations for ordination of species data. _Oecologia_ 129, 271–280 (2001). Article ADS PubMed Google Scholar * Lepš, J.
& Šmilauer, P. _Multivariate Analysis of Ecological Data Using CANOCO_ (Cambridge University Press, Cambridge, 2003). Book MATH Google Scholar * Simpson, G. Restricted permutations;
using the permute package. http://cran.r-project.org (2012). * Booth, D. B., Roy, A. H., Smith, B. & Capps, K. A. Global perspectives on the urban stream syndrome. _Freshw. Sci._ 35,
412–420 (2016). Article Google Scholar * Peipoch, M., Brauns, M., Hauer, F. R., Weitere, M. & Valett, H. M. Ecological simplification: Human influences on Riverscape complexity.
_Bioscience_ 65, 1057–1065 (2015). Article Google Scholar * Stoppe, N. D. C. _et al._ Worldwide phylogenetic group patterns of _Escherichia coli_ from commensal human and wastewater
treatment plant isolates. _Front. Microbiol._ 8, 2512 (2017). Article PubMed PubMed Central Google Scholar * Escobar-Páramo, P. _et al._ Large-scale population structure of human
commensal _Escherichia coli_ isolates. _Appl. Environ. Microbiol._ 70, 5698–5700 (2004). Article ADS PubMed PubMed Central CAS Google Scholar * Walk, S. T., Alm, E. W., Calhoun, L. M.,
Mladonicky, J. M. & Whittam, T. S. Genetic diversity and population structure of _Escherichia coli_ isolated from freshwater beaches. _Environ. Microbiol._ 9, 2274–2288 (2007). Article
PubMed Google Scholar * Touchon, M. _et al._ Phylogenetic background and habitat drive the genetic diversification of _Escherichia coli_. _PLoS Genet._ 16, e1008866 (2020). Article CAS
PubMed PubMed Central Google Scholar * R Core Team. _R: A Language and Environment for Statistical Computing_ (R Foundation for Statistical Computing, 2019). Google Scholar Download
references ACKNOWLEDGEMENTS This work was financed by grants from CONICET (PUE 22920160100122CO). MS is recipient of a doctoral fellowship from CONICET. We thank Dr. Inés O’Farrell and Dr.
Griselda Chaparro for their assistance during the sampling campaign, and one anonymous reviewer for helpful suggestions to improve variance partitioning analysis of phylogroup composition.
AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Instituto de Ecología, Genética y Evolución de Buenos Aires (IEGEBA), CONICET - Universidad de Buenos Aires, 1428, Buenos Aires, Argentina
Martín Saraceno & Martín Graziano * Departamento de Ecología, Genética y Evolución, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, 1428, Buenos Aires, Argentina
Martín Saraceno, Sebastián Gómez Lugo, Carmen A. Sabio y García, Nicolás Frankel & Martín Graziano * Instituto Nacional del Agua, 1804, Ezeiza, Argentina Nicolás Ortiz & Bárbara M.
Gómez * Instituto de Fisiología, Biología Molecular y Neurociencias (IFIBYNE), CONICET - Universidad de Buenos Aires, 1428, Buenos Aires, Argentina Nicolás Frankel Authors * Martín Saraceno
View author publications You can also search for this author inPubMed Google Scholar * Sebastián Gómez Lugo View author publications You can also search for this author inPubMed Google
Scholar * Nicolás Ortiz View author publications You can also search for this author inPubMed Google Scholar * Bárbara M. Gómez View author publications You can also search for this author
inPubMed Google Scholar * Carmen A. Sabio y García View author publications You can also search for this author inPubMed Google Scholar * Nicolás Frankel View author publications You can
also search for this author inPubMed Google Scholar * Martín Graziano View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS M.G. and M.S.
conceived the scope and the general design of the study. M.G., M.S., S.G.L. and C.S.G. were involved in the sampling campaign. M.G., M.S., S.G.L., C.S.G. and B.G. carried out analytical
determinations. N.F., M.G. and M.S. performed microbiological procedures. N.F., M.S., C.S.G. and S.G.L. performed molecular biology procedures and phylogroup identification. N.O. and M.S.
participated in the characterization of the watershed infrastructure. M.G. and M.S. contributed to data and statistical analysis. M.G. and M.S. were responsible for drafting the article and
figure design. All other authors provided critical input for the final manuscript. All authors approved the final version of the manuscript. CORRESPONDING AUTHOR Correspondence to Martín
Graziano. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under
a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate
credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article
are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons
licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of
this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Saraceno, M., Gómez Lugo, S., Ortiz, N. _et al._ Unraveling the
ecological processes modulating the population structure of _Escherichia coli_ in a highly polluted urban stream network. _Sci Rep_ 11, 14679 (2021).
https://doi.org/10.1038/s41598-021-94198-1 Download citation * Received: 29 December 2020 * Accepted: 07 July 2021 * Published: 19 July 2021 * DOI: https://doi.org/10.1038/s41598-021-94198-1
SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy
to clipboard Provided by the Springer Nature SharedIt content-sharing initiative