Sensorimotor inhibition during emotional processing
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Visual processing of emotional stimuli has been shown to engage complex cortical and subcortical networks, but it is still unclear how it affects sensorimotor integration processes.
To fill this gap, here, we used a TMS protocol named short-latency afferent inhibition (SAI), capturing sensorimotor interactions, while healthy participants were observing emotional body
language (EBL) and International Affective Picture System (IAPS) stimuli. Participants were presented with emotional (fear- and happiness-related) or non-emotional (neutral) EBL and IAPS
stimuli while SAI was tested at 120 ms and 300 ms after pictures presentation. At the earlier time point (120 ms), we found that fear-related EBL and IAPS stimuli selectively enhanced SAI as
indexed by the greater inhibitory effect of somatosensory afferents on motor excitability. Larger early SAI enhancement was associated with lower scores at the Behavioural Inhibition Scale
(BIS). At the later time point (300 ms), we found a generalized SAI decrease for all kind of stimuli (fear, happiness or neutral). Because the SAI index reflects integrative activity of
cholinergic sensorimotor circuits, our findings suggest greater sensitivity of such circuits during early (120 ms) processing of threat-related information. Moreover, the correlation with
BIS score may suggest increased attention and sensory vigilance in participants with greater anxiety-related dispositions. In conclusion, the results of this study show that sensorimotor
inhibition is rapidly enhanced while processing threatening stimuli and that SAI protocol might be a valuable option in evaluating emotional-motor interactions in physiological and
pathological conditions. SIMILAR CONTENT BEING VIEWED BY OTHERS NEUROPHYSIOLOGICAL EVIDENCE FOR EMOTIONAL ATTENTION MODULATION DEPENDING ON GOAL RELEVANCE Article Open access 08 April 2025
NEURONAL CORRELATES OF SPIDER PHOBIA IN A COMBINED FNIRS-EEG STUDY Article Open access 28 July 2020 THE EFFECTS OF SUBLIMINAL OR SUPRALIMINAL SADNESS INDUCTION ON THE SENSE OF BODY OWNERSHIP
AND THE ROLE OF DISSOCIATIVE SYMPTOMS Article Open access 15 November 2021 INTRODUCTION Appropriate motor responses to potential threats require rapid evaluation of the menace’s features
and the surrounding environment1. Being able to direct attention toward relevant sensory stimuli, as well as to correctly read emotional signals in others, is crucial in order to plan and
consequently choose the most appropriate reaction2,3,4,5. Threat-related visual stimuli are rapidly processed by the brain as shown by early modulations of event-related potentials
(ERPs)4,6,7; these stimuli are thought to increase sensory vigilance and grab attention2,8,9. In turn, perceived threats rapidly prime the body for action, as shown by both behavioural and
electrophysiological evidence10,11. Rapid motor responses to emotional stimuli have been often reported using motor-evoked potentials (MEPs) induced by transcranial magnetic stimulation
(TMS) of the primary motor cortex (M1), which offers the possibility to probe the motor system with high temporal resolution12,13,14. These studies have shown that emotional body language
(EBL)—and in particular, threat-related expressions, such as fearful body postures—modulate corticospinal excitability (CSE) and intracortical M1 processes such as intracortical facilitation
(ICF)12,13,14,15. Also, emotional stimuli like emotional facial expressions or more complex stimuli like natural emotional scenes from the International Affective Pictures System (IAPS)
have been shown to modulate CSE16,17,18,19,20,21,22. These studies have often reported two types of motor modulations, i.e., early inhibitory effects (i.e. when CSE or ICF was probed 70–150
ms after stimulus onset) that are thought to reflect early freezing-like orienting to the possible source of threat and later (> 150 ms) motor facilitations, reflecting increased motor
readiness to emotional stimuli12,15,18,20,23,24. Motor preparation requires fine tuning of sensorimotor interactions between somatosensory and motor regions25,26. However, despite the
relevance of sensorimotor integration to efficient action processing26,27,28, to date, little is known about how the underlying sensory-to-motor interactions are affected by emotional
information in humans. Sensorimotor system has been shown to be sensitive to emotions to the extent that emotional recognition processes are able to induce phenomena of motor mimicry and
somatosensory simulation, which activate the motor and the sensory system29,30. Furthermore, it has also been shown that these effects are more evident when the observer is confronted with
negative emotions (e.g., fear or anger) which are able to trigger similar internal emotional states and in turn enhance sensorimotor system’s readiness30. A valuable method to non-invasively
probe sensorimotor integration at the cortical level is the so called short-latency afferent inhibition (SAI) TMS protocol31 which involves pairing electrical stimulation of peripheral
somatosensory afferents with focal TMS targeting of the contralateral M1. Studies using the SAI protocol have shown that peripheral nerve stimulation at the contralateral wrist reduces the
amplitude of MEPs when the TMS pulse is given to M1 2–8 ms after the arrival of the afferent volley in cortex. The circuitry involved in SAI is complex. The sensory input exerts its
inhibitory effects on the corticospinal neurons through γ-Aminobutyric acid (GABA)-ergic intracortical circuits32,33, but its magnitude is also modulated by dopaminergic34 and cholinergic
neuromodulatory circuits35. As previously stated, although the exact mechanisms of SAI are still not clear, the contributions of several neurotransmitters in the phenomenon have been
studied. It has been shown by Di Lazzaro et al. that SAI was reduced (i.e., less sensorimotor inhibition) when subjects received benzodiazepines binding the α1-subunit of the GABAA receptors
(e.g., Zolpidem)36, while it was found to be increased after the administration of acetylcholinesterase inhibitors37. Thus, as suggested in a review by Turco et al., SAI generation is
probably related to specific inhibitory cells activated by cholinergic inputs38. It has been also shown that SAI was reduced in PD patients under l-Dopa medication in the ON phase, showing a
potential detrimental effect on sensorimotor inhibition of high concentration of Dopamine34. If we consider the fact that cholinergic efferent projections of the basal forebrain nuclei to
the cortex play a critical role in functions such as arousal, attention, cognitive and emotional processes39 and that SAI has been shown to be dynamically modulated by cognitive processes,
for instance working memory and attention40,41,42 and planning of both executed and imagined movements43,44, it appears plausible that it is a suitable tool to explore dynamic modulation of
cholinergic networks during higher-order functions such as emotional processing. Here, we designed two TMS experiments to assess SAI while healthy participants were observing fear-related
and happiness-related EBL and IAPS pictures and neutral controls. Our hypothesis is that processing emotional signals—which are known to affect perceptual and motor as well as cognitive
processes—would also influence sensorimotor integration. While prior work exploring early motor response to emotional stimuli have typically used EBL stimuli—which are known to induce
emotion-related but also motor resonance effects at the CSE level12,19,45,46—in the present research we tested both EBL and IAPS pictures so to assess the generalizability of the effects and
disentangle whether any modulation of the SAI protocol could be related to emotional modulation, motor resonance or other general effects. More specifically, we aimed to compare complex
emotional scenes, controlled in order to be embedded with minimal motor information, with emotional body postures, whom intrinsically carry both motor and emotional information, in order to
understand if the prevalence of one of these two types of information (i.e., emotional or motor) is responsible for the modulation of the sensorimotor network. Building on prior work showing
early and later changes in MEPs during emotional processing (e.g., Refs.12,13,23,47), here, we tested SAI at two different time-points, i.e., at 120 ms and 300 ms from picture onset (as
performed by Borgomaneri et al. in the studies12,23, respectively). While the 120 ms time point allows us to assess early subcortical-cortical automatic response to emotional stimuli12, the
300 ms timepoint allows assessing later, cognitive processing of motor and emotional information12,23. Building on the evidence that threat-related signals triggers early response in
cortico-subcortical networks involved in emotion processing and action planning48,49,50 and that early response to emotional signals—in particular to fear-related signals—may involve
inhibitory motor processes12,13,23, we expected that fear-related pictures would enhance SAI effects in the early time point. A control experiment was also performed to test whether a
simple, non-emotional, visual stimulus (i.e., while observing a black screen at rest) was able to modulate SAI at 120 and 300 ms differently from the SAI recorded at baseline. In this case,
we aimed to exclude that the modulation of sensorimotor inhibition could have been a consequence of the mere observation of a visual stimulus itself, possibly driven by light changes and not
by the increased attentional load derived from the observation of more complex emotional stimuli. Finally, we also considered the fact that motor reactivity during perception of social and
emotional information can be largely affected by inter-individual differences in specific personality traits22,51,52. In particular prior work has shown an association between behavioural
inhibition (BIS) and the magnitude of motor response to EBL, with larger M1 suppression due to fear-related EBL in participants showing higher scores at the BIS scale15. Therefore, we also
submitted participants to BIS and behavioural approach system (BAS)53,54. The BIS is described as an attentional system that disrupts ongoing behavioural response in order to allow the
subject to focus on relevant cues (e.g. punishment, non-reward or novelty), while the BAS is linked to positive feelings such as joy or optimism or negative such as rage and aggression; and
BIS/BAS scales have been used in several neuroscientific studies55,56. In this way, we tested whether interindividual differences in BIS/BAS predict magnitude of sensorimotor integration as
indexed by the SAI effects. METHODS PARTICIPANTS Thirty healthy participants (15 males, mean age ± SD 23.7 years ± 3.3) were enrolled in the main study (16 for the EBL experiment and 14 for
the IAPS experiment) and 10 additional participants (6 males, 28.3 years ± 2.1) took part in a control experiment in which sensorimotor modulation was investigated during observation of a
non-emotional visual stimulus (see “Visual stimuli”). In the main study, 16 participants (6 males, mean age ± SD 24.6 years ± 3.9) were tested with EBL stimuli and 14 participants (9 males,
mean age ± SD 22.5 years ± 2.3) were tested with IAPS stimuli. All participants were in good health, without any nervous, muscular, orthopaedic or cognitive disorders. Right arm dominance
was determined by means of the Edinburgh Handedness inventory57. Prior to the experimental session, all participants gave written informed consent and filled out a TMS safety, 13-items
screening questionnaire58. The experimental protocol was approved by the ethics committee of the University of Genoa and was carried out in agreement with legal requirements and
international norms stated in the adjourned declaration of Helsinki59. Considering the impossibility to retrieve previous experimental SAI data recorded in a similar study design, in the
main experiment we opted for a post-hoc power analysis in order to assess if the sample size we used in our study was appropriate. Results of the post-hoc power analysis showed that the
sample size used for the EBL experiment (N = 16) was appropriate with a power of 95% (1 − β = 0.95; α = 0.05; effect size = 0.97), while for the IAPS experiment (N = 14) we retrieved a power
of 94% (1 − β = 0.94; α = 0.05; effect size = 1.00). Power analysis was computed on G*Power 3.1. VISUAL STIMULI Visual stimuli were presented on a 22-inches computer screen (resolution 1680
× 1050, refresh rate 60.0 Hz; 16.67 ms ± 12.37 ms) located at 1.5 m away from the participants. Refresh rate was assessed via a photosensor and corresponded to normative values60. A total
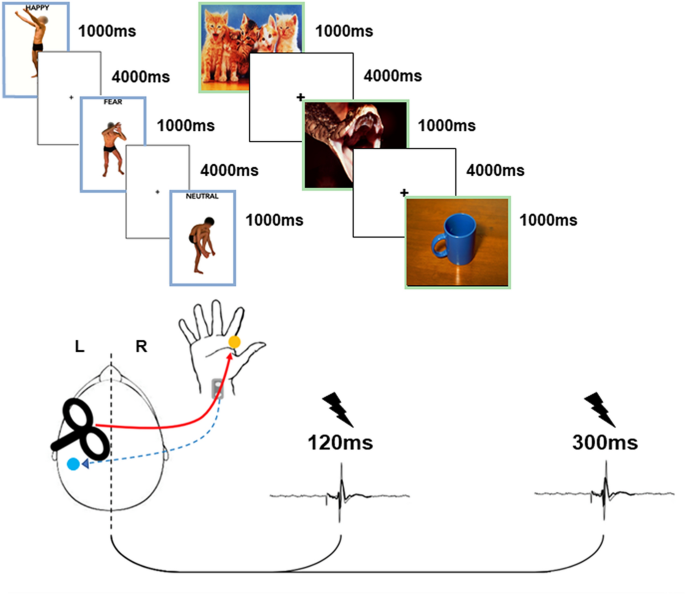
of 90 emotional visual stimuli were used for the main study, 45 for the EBL experiment and 45 for the IAPS experiment (see Supplementary Materials and Fig. 1). For the EBL experiment,
emotional posture pictures were selected from a validated EBL database12,19. EBL pictures depict four actors in different postures with emotional and neutral valence, thirty depicting
negative (fear) and positive (happiness) movements and fifteen with no emotional significance (neutral). The actors were not handling objects and their face was blanked-out. Regarding the
IAPS experiment, 45 stimuli were taken from the IAPS database, fifteen with negative emotional valence (fear), fifteen with positive valence (happiness) and fifteen neutral pictures
(neutral). Fear evoking IAPS pictures were extrapolated from the study of Barke and colleagues in order to select fear-related stimuli, not contaminated by other negative emotions (e.g.
disgust or rage)61. Furthermore, considering that the goal of the IAPS experiment was to test whether emotional scenes, with little or no motor information, were able to induce a modulation
in the sensorimotor circuit, we excluded all the IAPS pictures that depicted whole human bodies involved in some kind of actions. For the control experiment, the visual stimulus was a black
cross on a white screen, with controlled resolution and refresh rate as for the main experiment. Each experimental stimulus (EBL and IAPS pictures in the main experiment, black cross in the
control experiment) lasted 1000 ms, with an inter-stimulus interval of 4000 ms. TRANSCRANIAL MAGNETIC STIMULATION (TMS) Single-pulse TMS was delivered using a Magstim 200 stimulator
(Magstim, UK) with a monophasic current waveform connected to a figure-of-eight-shaped coil (external diameter of each loop, 9 cm) held tangentially to the scalp. The centre of the junction
of the coil was placed over the hand area of the contralateral M1 (i.e., left M1) at the optimal position (hotspot) to elicit MEPs in the right first dorsal interosseous (FDI), with the
handle pointing backwards and a 45° away from the midline. With this coil orientation, the induced current flowed in an anterior–medial direction approximately perpendicular to the central
sulcus. The optimal coil location was searched by slightly moving the coil over the left M1 area until MEPs of maximal amplitude and lowest threshold in the right FDI were elicited. The
exact coil position was marked by an inking pen to ensure an accurate positioning of the coil throughout the experiment. SHORT LATENCY AFFERENT INHIBITION (SAI) PROTOCOL SAI was tested
following the validated protocol by Tokimura and colleagues31. The SAI effect was tested with a supra-threshold test TMS stimulus over the M1 representation of right FDI adjusted to produce
MEPs of ~ 1 mV (peak-to-peak amplitude), preceded (20 ms inter-stimulus interval) by an electrical conditioning stimulus over the contralateral median nerve at the right wrist. This
conditioning interval was shown to be optimal to evoke MEP inhibition in several studies31,62. Electrical stimulation (ES) was delivered through a bipolar electrode (cathode proximal) using
a square-wave pulse (duration, 200 μs) set at intensity just above the threshold for evoking a small twitch in the _opponens pollicis_ muscle (Digitimer D180 high voltage electric
stimulator). In each experiment (EBL, IAPS) of the main study, a total of 90 trials were performed, of which 45 were conditioned trials (COND: ES + TMS) and 45 unconditioned trials (TEST:
TMS). In the control experiment, a total of 30 trials were performed (15 TEST and 15 COND). For all conditions, in the main experiment and in the control experiment, SAI was tested at two
time-intervals: 120 ms and 300 ms after visual stimulus onset. Additionally, two baselines recording of 30 trials each were recorded before (PRE) and after (POST) the experimental session.
ELECTROMYOGRAPHIC (EMG) RECORDING EMG was recorded with silver disc surface electrodes placed with a belly-tendon montage over the right FDI muscle. Electromyography signals were amplified
and filtered (20 Hz to 1 kHz) with a D360 amplifier (Digitimer). The signals were sampled at 5000 Hz, digitized with a laboratory interface (Power 1401, Cambridge Electronic Design,
Cambridge, UK) and stored on a personal computer for display and later offline data analysis. EXPERIMENTAL PROCEDURES In all experiments, participants were comfortably sitting on a chair at
a distance of approximately 1.5 m from the computer screen where the visual emotional or neutral stimuli (main experiment) or the non-emotional stimulus (control experiment) were presented.
The experiment was programmed using Matlab (version 9.9.0.1467703, MathWorks, Natick, MA, USA) software to control picture presentation and to trigger ES and TMS. MEPs were collected in 4
blocks (two baseline blocks and two experimental blocks). The baseline blocks were the first (PRE) and the last (POST) (30 trials each): participants were asked to keep their eyes open with
the instruction to watch a black screen while receiving TMS over M1 (interpulse interval 4000 ms). The interpulse interval was determined following the Guidelines of the International
Federation of Clinical Neurophysiology63. Fifteen COND trials and 15 TEST trials were collected in each block. In the experimental blocks (90 trials each) participants were asked to stay
still and focus on the pictures sliding on the screen. In these two blocks, TMS pulse was delivered at 120 or 300 ms after picture onset and the order of the blocks was randomized. The
trials in the experimental blocks were divided in 45 COND trials and 45 TEST trials, which in turn provided for 30 emotional stimuli (15 for fear and 15 for happiness) and 15 neutral stimuli
each. Participants were asked to stop the experiments whenever they felt discomfort or pain. They were also invited to maintain attention to the stimuli at regular intervals throughout the
experiment and a verbal comment was asked at the end of each trial were all participants had to tell if they perceived any emotion on the observed pictures. A pause of 3–4 min between blocks
was allowed to prevent fatigue or lack of attention. The experiment lasted for approximately 120 min. A similar protocol was used for the control experiment, where 30 trials (15 COND and 15
TEST) were performed for the two baseline blocks (PRE and POST) and 30 trials were performed at 120 and 300 ms after stimulus onset. Finally, the Italian version of the BIS and BAS
questionnaire was submitted via email to all main experiment’s participants in order to acquire individuals’ specific personality dimensions54. The whole questionnaire consists of 20 items
of which the firsts seven give the BIS score (maximum 35 points) and the remaining give the BAS score (maximum 65 points). The BAS scale is divided in three main subclasses as follow: BAS
Reward from item 8 to item 12, BAS Drive from item 13 to item 16 and BAS Fun from item 17 to item 20. The subject had to give a rating from 1 to 5, where 1 stood for “It doesn’t describe me
at all” and 5 stood for “It describes me completely”. A high score in the BIS scale indicates an increased propensity in withdrawal or inhibitory behaviour when facing a potential threat,
while a high score in the BAS scale is an indication of action engagement55,64. DATA ANALYSIS Neurophysiological data were analysed offline. The mean peak-to-peak MEP amplitude (in mV) was
computed for each condition, with or without peripheral stimulation (MEPs COND and MEPs TEST). To evaluate the SAI effect, conditioned MEPs were expressed relative to unconditioned MEPs (SAI
= COND/TEST). Moreover, SAI value at each testing time (120 and 300 ms) was normalized to SAI recorded at baseline (SAI Ratio = SAIExperiment/SAIBaseline). This normalization shows data as
an increased or decreased SAI compared to the SAI recorded at baseline. A value greater than 1 indicates a SAI reduction in relation to baseline (i.e., reduced sensorimotor inhibition),
whereas a value lower than 1 indicates an increase of SAI in relation to baseline (i.e., increased sensorimotor inhibition)44,65. The same analysis was run also for the data acquired in the
control experiment. STATISTICAL ANALYSIS Before performing further statistical analysis, we checked that all variables were normally distributed (Shapiro–Wilk test) and that sphericity was
respected (Mauchly’s test). Levene’s test of homogeneity of variances was also performed in order to assess the comparability of the two datasets in the main experiment (EBL and IAPS). The
effects of electrical conditioning on MEP amplitudes in the two baseline blocks were tested using a mixed factors ANOVA with BLOCK (PRE and POST) and TYPE OF STIMULATION (TEST and COND) as
within-subjects factors and GROUP (EBL and IAPS) as between-subjects factor. For the main study, SAI Ratio data were analysed via a 3 × 2 × 2 mixed factors ANOVA, with EMOTION (fear,
happiness and neutral) and TIME (120 ms and 300 ms) as within-subject factors and GROUP (EBL and IAPS) as between-subject effect. Post-hoc analysis was performed by means of Bonferroni
correction method for multiple comparison. We also performed a 3 × 2 × 2 mixed factors ANOVA, with the same within-subjects factors (EMOTION and TIME) and GENDER (Male and Female) as
between-subjects effect, for both EBL and IAPS stimuli. In order to assess whether there was a difference between the unconditioned MEPs amplitudes (MEPs TEST) and SAI effects (COND/TEST)
recorded while processing EBL and IAPS pictures and the ones recorded at baseline, we performed a 3 × 2 mixed factors ANOVAs with CONDITION (Baseline, 120 ms and 300 ms) as within-subjects
factor and GROUP (EBLs and IAPS) as between-subjects factor separately for each emotion (fear, happiness and neutral). We opted to name differently the TIME and CONDITION factors in order to
highlight the presence of baseline data in CONDITION. Bonferroni corrected post-hoc tests were used to test significant effects. In the control experiment, MEPs TEST and SAI data were
analysed via a repeated measure ANOVA with CONDITION (Baseline, 120 ms and 300 ms) as within-subjects factor. Finally, to investigate a possible relationship between SAI modulation and
personality trait in relation to emotional processing, we performed a Pearson correlation between BIS/BAS questionnaire scores and SAI Ratio for normal distributed data and otherwise a
Spearman’s Rho correlation test was run. Statistical analysis was performed with SPSS 25. p-values of 0.05 were considered as threshold for statistical significance. RESULTS At baseline,
when TMS was preceded by ES, MEPs amplitude consistently decreased relatively to TMS alone (Table 1), thus replicating the typical SAI effects. Accordingly, the statistical analysis showed a
strong effect of TYPE OF STIMULATION (F [1, 28 = 324.567; p < 0.01; pη2 = 0.921), with lower amplitude in the COND condition (mean ± SEM = 0.468 ± 0.027) compared to the TEST condition
(1.27 ± 0.048), while no influence of the factors BLOCK or GROUP were found (all F ≤ 2.23; all p > 0.05), thus suggesting no change in motor excitability over time in the two experimental
groups submitted to the observation of EBL and IAPS stimuli. Furthermore, considering that no influence of BLOCK was observed, indicating no changes in motor excitability over time, the
baseline blocks were averaged for computing the SAI Ratio (see next section). MAIN EXPERIMENT MODULATION OF SENSORIMOTOR INTEGRATION DURING PERCEPTION OF EMOTIONAL PICTURES: COMPARISON
BETWEEN EMOTIONS Statistical analysis on SAI ratio values showed that sensorimotor modulation occurred at 120 ms specifically for fearful stimuli and at 300 ms for all the emotional stimuli
(see Fig. 2). ANOVA on SAI Ratio data showed significant results for both main effects EMOTION (F [2, 56] = 9.723; p < 0.01; pη2 = 0.258) and TIME (F [1, 28] = 17.709; p < 0.01; pη2 =
0.387), which were qualified by the interaction effect EMOTION × TIME (F [2, 56] = 4.079; p = 0.035; pη2 = 0.127). All the effects involving the factor GROUP were not significant in the
ANOVA (all F ≤ 0.508; all p > 0.05), suggesting comparable sensorimotor modulations with the two sets of pictures. Post-hoc analysis of the EMOTION × TIME interaction showed that only
during processing of fearful stimuli at 120 ms from the stimulus onset SAI increased (i.e., larger inhibition) respect to the same timing for happiness (120 ms, p < 0.01) and neutral (120
ms, p < 0.01). Furthermore, only for fear and happiness, SAI recorded at 120 ms after stimulus onset was significantly higher compared with SAI recorded at 300 ms (p < 0.01 for fear;
p = 0.012 for happiness). No effects for GENDER was retrieved in the analysis for EBL or IAPS stimuli (all F ≤ 0.508; all p > 0.05). MODULATION OF CORTICOSPINAL EXCITABILITY AND
SENSORIMOTOR INTEGRATION DURING PERCEPTION OF EMOTIONAL PICTURES Considering that the analysis on SAI Ratio data showed an influence of EMOTION, which was different for the early and later
time points considered (i.e., EMOTION × TIME interaction) we further investigated SAI modulation during observation of emotional stimuli performing additional analyses. The ANOVAs on MEPs
TEST showed no significant main effects or interaction of the factors CONDITION or GROUP across the three emotions (fear, happiness and neutral; all F ≤ 1; all p > 0.05). In contrast, the
ANOVA on SAI data for fear showed significant effect of CONDITION (F [2, 56] = 28.814; p < 0.01; pη2 = 0.507), but no main effect or interaction involving the factor GROUP (p > 0.05).
Post-hoc analysis (see Fig. 3A) showed that the SAI effect increased (i.e., lower SAI values) for fearful stimuli in the early time point (120 ms) relative to the later time point (300 ms)
and baseline (all p < 0.01). Furthermore, at 300 ms after fearful stimuli onset, SAI was significantly lower relative to baseline (p < 0.01). The ANOVA on SAI effects for the other two
conditions (Happiness, Fig. 3B; Neutral stimuli, Fig. 3C) showed a significant effect of CONDITION for both happiness (F [2, 56] = 9.669; p < 0.01; pη2 = 0.257) and neutral stimuli (F
[2, 56] = 5.824; p < 0.01; pη2 = 0.172), but not main effect or interaction involving the factor GROUP (all F ≤ 1; all p > 0.05). Post-hoc analysis showed that for both
happiness-related and neutral stimuli, SAI significantly decreased at 300 ms relative baseline levels (all p < 0.01) and showed similar trend relative to the 120 ms condition
(happiness-related stimuli: p = 0.016; neutral stimuli p = 0.082). CORRELATION ANALYSIS Twenty-one out of 30 participants accepted to compile the BIS/BAS questionnaires and were included in
the correlation analysis. Three of them were excluded as outliers (distance from the trend-line higher than 2.5 standard deviations), for a total of 18 participants from both the EBL and the
IAPS experiments. Since, statistical analysis on SAI Ratio data did not show any significant difference between groups (EBL and IAPS), before running the correlation analysis, we performed
a linear regression analysis to verify that the GROUP was not a significant predictor for BIS, BAS and SAI RATIO data. Statistical analysis showed that GROUP was never a significant
predictor (Supplementary Information), thus we run the correlation analysis combining the data of EBL and IAPS conditions. Pearson correlation was run separately for the BIS and BAS
questionnaire scores that presented normal distribution. Results showed that the only significant correlation we found was a positive correlation between BIS and SAI Ratio data for fearful
stimuli at 120 ms post-stimulus (p = 0.003, r = 0.627) (Fig. 4). Correction for multiple comparisons was run in accordance with the following formula from the work by Curtin and Schulz66:
$${\alpha }^{{\prime}}=1-{\left(1-\alpha \right)}^{\frac{1}{\mathrm{k}}},$$ where α′ will be the new alpha-value corrected for multiple comparisons, α is the value for significance
previously set in our study and _k_ is the number of comparisons on the same dependent variable (which in our case is equal to 10). Therefore, the new alpha-value is set at 0.005.
Acknowledged that the p-value computed for the correlation between the BIS value and the SAI Ratio data for fearful stimuli at 120 ms post-stimulus is still significant after multiple
comparisons correction (p = 0.003), we can say that the positive correlations we found indicates that participants with augmented SAI at 120 ms from the picture onset had a lower BIS score.
All other correlations for the BIS score, as well for the BAS score, were not significant (all p > 0.05). CONTROL EXPERIMENT Statistical analyses on MEPs TEST and SAI data for the control
experiment showed no significant differences for CONDITION (p > 0.05), meaning that all MEPs and their relative SAI during the observation of a non-emotional visual stimulus were
comparable at baseline and at 120 ms or 300 ms after the stimulus onset (see Table 2). DISCUSSION The main aim of this study was to investigate sensorimotor integration in healthy adults
while observing EBL and IAPS pictures. Furthermore, we explored whether interindividual differences in BIS/BAS personality traits predict the magnitude of sensorimotor integration mechanisms
as tapped by the SAI effect. The results showed a consistent SAI effect on single pulse TMS when ES was administered over the median nerve. Remarkably, we observed the following sets of
novel findings: (i) only while processing fearful stimuli, sensorimotor inhibition (i.e., the SAI effect) increased in magnitude, at earlier latencies (120 ms after the stimulus onset)
irrespective of the type of picture (EBL or IAPS); (ii) at 300 ms after the stimulus onset, sensorimotor inhibition decreased while processing all kind of emotional stimuli (fear, happiness,
neutral) again irrespective of the type of picture (EBL or IAPS); (iii) lastly, there was a correlation between sensorimotor inhibition at earlier latencies (120 ms after the stimulus
onset) while processing fearful stimuli and BIS score. There are different possible explanations accounting for the enhanced SAI effects—that is, increased sensorimotor inhibition—observed
at 120 ms after the stimulus onset only for fearful stimuli, and these accounts deal with modulation of sensory afference and activity in the attentional circuits. Indeed, the circuitry
underlying SAI is complex. A recent neuronal model of SAI proposes that sensory afference drives corticospinal activity through a perisomatic inhibitory projection as well as the modulation
of distal dendritic excitatory input from the I-wave generators38. The perisomatic inhibitory input is proposed to originate from ɣ-amino butyric acid (GABA) basket cells located in layer IV
of the motor cortex. These basket cells are hypothesized to receive excitatory input from somatosensory pyramidal neurons as well as from the thalamus and are sensitive to cholinergic
inputs coming from the basal forebrain (BF)67,68. The BF cholinergic system has extensive diffuse projections to neocortex as well as projections to and from basolateral amygdala and
olfactory bulb and is strongly implicated in attention11,39. Studies suggest that increased sensorimotor inhibition may derive from an augmentation of the sensory afferent volleys to the
cortex. Indeed, it has been consistently shown that the greater the afferent volley evoked by peripheral stimulation, the stronger is the magnitude of SAI (for a review see Ref.38). However,
modulation of sensorimotor inhibition may also stem from the engagement of attentional circuits. Indeed, paying attention to the body part tested in the SAI protocol (hand) during an
attentional task (internal focus) leads to a greater SAI with respect to what observed when the focus is external69. Such an effect was explained by observing that internal focus might
result in an augmentation of somatosensory volleys from the specific body part, which is in keeping with the notion that observing and paying attention to a specific body part enhance the
somatosensory representation of that body part70. In a similar vein, observing a touch onto another body—which is known to engage the activation of the somatosensory cortex through a
somatosensory resonance mechanism71,72, also increase the SAI effect73. On the other hand, SAI effects may also be modulated by more general attentional mechanisms as well. In a recent
study, increase of SAI was observed during a computerized non-verbal recognition memory task that requires recognizing previously encoded faces, in the retrieval phase40. The authors
proposed that the retrieval phase of the visual memory task could evoke a considerable activation of widespread cholinergic projections from the BF to the cortex40. In a more speculative
way, we can also ask ourselves what is the structure that may theoretically drive these mechanisms (modulation of sensory afference and/or recruitment of attentional circuits) during
processing of emotional fearful aversive stimuli? Although in our study we cannot directly address this issue, we know that a pivotal structure in decoding emotional information,
specifically for threat-related stimuli, with widespread cortical and subcortical interactions is the amygdala (AMG). AMG has been shown to receive complex sensory information from the
periphery and process them in its lateral and basolateral nuclei, which in turn project to its central nucleus74. Then, the central nucleus of the AMG sends projections to a widespread
network involving a multitude of structures which are involved in both motor and non-motor responses at short and long latencies3,4,75,76. More specifically, in response to aversive stimuli,
the central nucleus of the AMG has been shown to project to several subcortical structures involved in attentional processes such as the BF and the locus coeruleus. Through its projections
toward cholinergic nuclei located in the BF, AMG is able to influence an extended cortical and subcortical network which in turn increases the attentional control over a stimulus3,39.
Furthermore, this widespread cholinergic activity might be able not only to increase attention, but also to lower the threshold of the sensory system in order to focus attentional resources
over threat-related stimuli and consequently potentiate sensory afferences processing39. Projections from the central nucleus of the AMG have also an influence over the noradrenergic cells
of the locus coeruleus, structure responsible for stress and conditioned fear response as well as for autonomic activity39,77,78. Taken all together, the engagement of cholinergic circuits
secondary to emotional stimulation coupled together with the activation of the locus coeruleus and the concomitant activation of the sensory thalamus may lead to an increased state of
vigilance which directs attention toward strongly arousing stimuli with aversive properties3,11,39,77. Related to the temporal dimension of the activation of the AMG, it has been shown that
the AMG promptly reacts to fearful signals, for example showing a short-latency activation at already ~ 70 ms from presentation of a fearful face—which might be linked to an evolutionary
mechanism for processing threat-related stimuli, in order to rapidly react to a potential menace79. Such a mechanism was referred as ‘preparedness’, which is defined as an instinctive
response to known aversive stimuli that does not show an elaborated cognitive processing of the afferent information80. So far this phenomenon has been described in processing emotional
facial expressions79, but since subcortical areas activated during facial expression processing are partially overlapping with those engaged during processing of EBL and IAPS (one over all
is AMG), it is plausible to expect similar pattern of activation for both categories of emotional stimuli6,81,82. Based on the aforesaid premises and on our results, it appears reasonable to
speculate that the rapid amygdala activation, as described for fearful facial expressions, might be a key component for the sensorimotor modulation we found for fearful pictures, acting via
the same principle of ‘preparedness’ observed for threat-related faces, even though specific imaging studies are needed in order to confirm these supposed mechanisms. Moreover, the fact
that we observed such a fast increase of SAI for all fearful pictures, being them EBL or IAPS, might be related to the fact that an aversive emotional content is able to rapidly activate a
vast and intertwined network capable of focusing attentional resources over a potential threat via the induction of sensory vigilance and hence inducing a transient and short-latency
freezing-like phenomenon of the motor cortex12,79. The second result of our study was that at longer latency (i.e., 300 ms after the stimulus onset) sensorimotor inhibition decreased while
processing all kind of emotional stimuli (fear, happiness, neutral) conveyed by all the pictures (EBL and IAPS). First, it is worthy to note that in our control experiment, planned to test
SAI at 120 and 300 ms after the onset of a picture representing a black cross on a white screen, we did not find a modulation of SAI. This result mostly excludes that solely being presented
with a visual stimulus shown on a screen may modulate sensorimotor inhibition (for a detailed analysis on the topic see “Limitations”). As an example of sensorimotor modulation derived from
higher order cognitive processes, a decreased sensorimotor inhibition has been observed during a verbal working memory task, with increasing memory set size42. Indeed, SAI was significantly
reduced during memory maintenance of six-digit compared to two-digit set42. This result was interpreted as the necessity to increase the suppression of the task-irrelevant somatosensory
afferent projections to motor cortex during the maintenance period of the verbal working memory task. Following this finding, here we showed no modulation during visual processing of a blank
screen, but when the visual information increased in complexity, as for IAPS and EBL pictures, SAI decreased at 300 ms. We might propose that even during passive visual processing of images
of different complexity, decreased sensorimotor inhibition may represents the suppression of the task-irrelevant somatosensory afferent projections to motor cortex, similarly to what
happens during a higher order cognitive processing such as a visual working memory task of increasing complexity. Indeed, 300 ms latency is consistent with cognitive processing of the visual
stimuli. Event-related potentials studies showed during visual processing of a variety of stimuli a consistent component related to cognitive control, that is the P300, a positive wave that
occurs at around 300–450 ms after stimulus presentation and which is maximal over the central-parietal region83,84. Major determinants of P300 amplitude during visual processing are reward
value, affective significance and the influence of these factors on attention resource allocation9,85,86. The third result of our study was that sensorimotor modulation during processing of
fearful stimuli at 120 ms correlated with the BIS score: the more the increase in sensorimotor inhibition at 120 ms while processing fearful stimuli, the lower the BIS score. A low score in
the BIS questionnaire indicates an individual minor propensity in withdrawal behaviour when facing a potential threat. If modulation of sensorimotor inhibition is driven by the activation of
the AMG orchestrating a cascade of events including increased attention with higher sensory vigilance, we can hypothesize that these processes are linked to behaviour, including propensity
in withdrawal behaviour. Greater attention and sensory vigilance may guide a higher propensity in facing a potential threat, rather than withdraw from it. This speculation is in line with
recent behavioural findings of our group: by using the same sets of EBL and IAPS images adopted here and analysing response time in a recognition task, we found that response time for fear
pictures were lower than for happy and neutral, particularly for EBL pictures24. Possible explanations for this finding may lie on shorter time for processing fear respect to happy pictures,
but also on fear stimuli facilitating faster motor commands with respect to happy and neutral. In any case it is important to note that, although the results of the correlation analysis
resulted significant for multiple comparisons correction, deeper investigations are needed in order to confirm our findings with a bigger sample size and separately for EBL and IAPS.
LIMITATIONS Some limitations of this study deserve to be discussed. First, we a priori decided to explore sensorimotor inhibition only at 120 ms and 300 ms from picture onset, in order to
test for a rapid-automatic sensorimotor modulation and for cognitive processing of EBL and IAPS. However, in future studies it will be of interest to study the temporal dynamics of
sensorimotor inhibition more in the details analysing, for instance, whether an automatic process starts early for EBL or IAPS pictures and how long it lasts. Second, future studies may
explore whether sensory gating or sensory augmentation mechanisms are involved, by the analysis of somatosensory evoked potentials. Moreover, a more structured online control task in order
to verify that the participants were paying attention to the visual stimuli sliding on the screen (e.g., question about the emotional content of the observed stimulus between trials) might
be of use in order to eliminate a possible bias regarding attentional orientation. It would also be of interest a control on visual orientation, via an eye-tracking system, able to assess
whether sensorimotor inhibition in EBL is linked to the observation of a specific body part (e.g., hands) or to the body posture as a whole. We also have to consider as a limitation the
limited sample size used in the control experiment. Whether sensorimotor inhibition is not modulated by simple, non-emotional stimuli but only by complex images more attentional engaging, is
worthy to be confirmed in a larger sample size. Finally, the role of cholinergic circuits may be addressed by exploring this process in pathological conditions with cortical cholinergic
denervation (i.e., Alzheimer’s disease) or under pharmacological modulation of cholinergic activity. CONCLUSIONS Here we showed that sensorimotor inhibition, depending on the magnitude of
afferent sensory volley and on the activity of cholinergic circuits, is dynamically modulated during visual processing of emotional stimuli. At short latency from picture onset, reflecting
automatic processing of emotional information, only negative, fearful stimuli induced a modulation of sensorimotor inhibition. At 300 ms from picture onset, reflecting cognitive appraisal of
visual information, SAI appeared to decrease in relation to the complexity of the image, rather than its emotional content. Sensorimotor inhibition has been used so far as a tool to explore
cognitive-motor interaction, but the results of this study suggest that it may also be used to explore emotional-motor interaction, in physiological and pathological conditions. DATA
AVAILABILITY The data that support the findings of this study are available from the corresponding author upon reasonable request. REFERENCES * Gladwin, T. E., Hashemi, M. M., van Ast, V.
& Roelofs, K. Ready and waiting: Freezing as active action preparation under threat. _Neurosci. Lett._ 619, 182–188 (2016). Article CAS PubMed Google Scholar * Smith, N. K.,
Cacioppo, J. T., Larsen, J. T. & Chartrand, T. L. May I have your attention, please: Electrocortical responses to positive and negative stimuli. _Neuropsychologia_ 41, 171–183 (2003).
Article PubMed Google Scholar * Vuilleumier, P. How brains beware: Neural mechanisms of emotional attention. _Trends Cogn. Sci._ 9, 585–594 (2005). Article PubMed Google Scholar * van
Heijnsbergen, C. C. R. J., Meeren, H. K. M., Grèzes, J. & de Gelder, B. Rapid detection of fear in body expressions, an ERP study. _Brain Res._
https://doi.org/10.1016/j.brainres.2007.09.093 (2007). Article PubMed Google Scholar * Roelofs, K. Freeze for action: Neurobiological mechanisms in animal and human freezing. _Philos.
Trans. R. Soc. B Biol. Sci._ 372, 20160206 (2017). Article CAS Google Scholar * De Gelder, B. Why bodies? Twelve reasons for including bodily expressions in affective neuroscience.
_Philos. Trans. R. Soc. B Biol. Sci._ 364, 3475–3484 (2009). Article Google Scholar * Meeren, H. K. M., Van Heijnsbergen, C. C. R. J. & De Gelder, B. Rapid perceptual integration of
facial expression and emotional body language. _Proc. Natl. Acad. Sci. U. S. A._ 102, 16518–16523 (2005). Article CAS PubMed PubMed Central ADS Google Scholar * Schupp, H. T.,
Junghöfer, M., Weike, A. I. & Hamm, A. O. Attention and emotion: An ERP analysis of facilitated emotional stimulus processing. _NeuroReport_ 14, 1107–1110 (2003). Article PubMed Google
Scholar * Olofsson, J. K. & Polich, J. Affective visual event-related potentials: Arousal, repetition, and time-on-task. _Biol. Psychol._ 75, 101–108 (2007). Article PubMed PubMed
Central Google Scholar * Frijda, N. H. Impulsive action and motivation. _Biol. Psychol._ 84, 570–579 (2010). Article PubMed Google Scholar * Tamietto, M. & De Gelder, B. Neural
bases of the non-conscious perception of emotional signals. _Nat. Rev. Neurosci._ 11, 697–709 (2010). Article CAS PubMed Google Scholar * Borgomaneri, S., Vitale, F., Gazzola, V. &
Avenanti, A. Seeing fearful body language rapidly freezes the observer’s motor cortex. _Cortex_ 65, 232–245 (2015). Article PubMed Google Scholar * Borgomaneri, S., Vitale, F. &
Avenanti, A. Early changes in corticospinal excitability when seeing fearful body expressions. _Sci. Rep._ 5, 1–9 (2015). Article CAS Google Scholar * Borgomaneri, S., Vitale, F. &
Avenanti, A. Early motor reactivity to observed human body postures is affected by body expression, not gender. _Neuropsychologia_ 146, 107541 (2020). Article PubMed Google Scholar *
Borgomaneri, S., Vitale, F. & Avenanti, A. Behavioral inhibition system sensitivity enhances motor cortex suppression when watching fearful body expressions. _Brain Struct. Funct._ 222,
3267–3282 (2017). Article PubMed Google Scholar * Lang Bradley, M. M. & Cuthbert, B. N. P. J. International affective picture system (IAPS): Affective ratings of pictures and
instruction manual. (2008). * Ekman, P. & Rosenberg, E. _What the Face Reveals: Basic and Applied Studies of Spontaneous Expression Using the Facial Action Coding System (FACS)_. (2005).
* Schutter, D. J. L. G., Hofman, D. & Van Honk, J. Fearful faces selectively increase corticospinal motor tract excitability: A transcranial magnetic stimulation study.
_Psychophysiology_ 45, 345–348 (2008). Article PubMed Google Scholar * Borgomaneri, S., Gazzola, V. & Avenanti, A. Motor mapping of implied actions during perception of emotional body
language. _Brain Stimul._ 5, 70–76 (2012). Article PubMed Google Scholar * Coelho, C. M., Lipp, O. V., Marinovic, W., Wallis, G. & Riek, S. Increased corticospinal excitability
induced by unpleasant visual stimuli. _Neurosci. Lett._ 481, 135–138 (2010). Article CAS PubMed Google Scholar * Borgomaneri, S., Gazzola, V. & Avenanti, A. Temporal dynamics of
motor cortex excitability during perception of natural emotional scenes. _Soc. Cogn. Affect. Neurosci._ 9, 1451–1457 (2014). Article PubMed Google Scholar * Borgomaneri, S., Vitale, F.,
Battaglia, S. & Avenanti, A. Early right motor cortex response to happy and fearful facial expressions: A tms motor-evoked potential study. _Brain Sci._ 11, 1203 (2021). Article PubMed
PubMed Central Google Scholar * Borgomaneri, S., Gazzola, V. & Avenanti, A. Transcranial magnetic stimulation reveals two functionally distinct stages of motor cortex involvement
during perception of emotional body language. _Brain Struct. Funct._ 220, 2765–2781 (2015). Article PubMed Google Scholar * Botta, A., Lagravinese, G., Bove, M., Avenanti, A. &
Avanzino, L. Modulation of response times during processing of emotional body language. _Front. Psychol._ 12, 1–11 (2021). Article Google Scholar * Gale, D. J., Flanagan, J. R. &
Gallivan, J. P. Human somatosensory cortex is modulated during motor planning. _J. Neurosci._ https://doi.org/10.1523/jneurosci.0342-21.2021 (2021). Article PubMed PubMed Central Google
Scholar * Rens, G. _et al._ Sensorimotor expectations bias motor resonance during observation of object lifting: The causal role of pSTS. _J. Neurosci._ 40, 3995–4009 (2020). Article CAS
PubMed PubMed Central Google Scholar * Catmur, C., Walsh, V. & Heyes, C. Sensorimotor learning configures the human mirror system. _Curr. Biol._ 17, 1527–1531 (2007). Article CAS
PubMed Google Scholar * Maffongelli, L. _et al._ Role of sensorimotor areas in early detection of motor errors: An EEG and TMS study. _Behav. Brain Res._ 378, 112248 (2020). Article
PubMed Google Scholar * Huis In’t Veld, E. M. J., van Boxtel, G. J. M. & de Gelder, B. The body action coding system II: Muscle activations during the perception and expression of
emotion. _Front. Behav. Neurosci._ 8, 330 (2014). Google Scholar * Ross, P. & Atkinson, A. P. Expanding simulation models of emotional understanding: The case for different modalities,
body-state simulation prominence, and developmental trajectories. _Front. Psychol._ 11, 309 (2020). Article PubMed PubMed Central Google Scholar * Tokimura, H. _et al._ Short latency
inhibition of human hand motor cortex by somatosensory input from the hand. _J. Physiol._ 523(Pt 2), 503–513 (2000). Article CAS PubMed PubMed Central Google Scholar * Di Lazzaro, V.
_et al._ Effects of lorazepam on short latency afferent inhibition and short latency intracortical inhibition in humans. _J. Physiol._ 564, 661–668 (2005). Article PubMed PubMed Central
CAS Google Scholar * Di Lazzaro, V. & Ziemann, U. The contribution of transcranial magnetic stimulation in the functional evaluation of microcircuits in human motor cortex. _Front.
Neural Circuits_ https://doi.org/10.3389/fncir.2013.00018 (2013). Article PubMed PubMed Central Google Scholar * Sailer, A. _et al._ Short and long latency afferent inhibition in
Parkinson’s disease. _Brain_ 126, 1883–1894 (2003). Article PubMed Google Scholar * Di Lazzaro, V. _et al._ Muscarinic receptor blockade has differential effects on the excitability of
intracortical circuits in the human motor cortex. _Exp. Brain Res._ 135, 455–461 (2000). Article PubMed Google Scholar * Di Lazzaro, V. _et al._ Segregating two inhibitory circuits in
human motor cortex at the level of GABAA receptor subtypes: A TMS study. _Clin. Neurophysiol._ 118, 2207–2214 (2007). Article PubMed CAS Google Scholar * Lazzaro, D. _et al._
Neurophysiological predictors of long term response to AChE inhibitors in AD patients. _J. Neurol. Neurosurg. Psychiatry_ 76, 1064–1069 (2005). Article PubMed Google Scholar * Turco, C.
V. _et al._ Short- and long-latency afferent inhibition; uses, mechanisms and influencing factors. _Brain Stimul._ 11, 59–74 (2018). Article PubMed Google Scholar * Davis, M. &
Whalen, P. J. The amygdala: Vigilance and emotion. _Mol. Psychiatry_ 6, 13–34 (2001). Article CAS PubMed Google Scholar * Bonnì, S., Ponzo, V., Di Lorenzo, F., Caltagirone, C. &
Koch, G. Real-time activation of central cholinergic circuits during recognition memory. _Eur. J. Neurosci._ 45, 1485–1489 (2017). Article PubMed Google Scholar * Mirdamadi, J. L.,
Suzuki, L. Y. & Meehan, S. K. Attention modulates specific motor cortical circuits recruited by transcranial magnetic stimulation. _Neuroscience_ 359, 151–158 (2017). Article CAS
PubMed Google Scholar * Suzuki, L. Y. & Meehan, S. K. Verbal working memory modulates afferent circuits in motor cortex. _Eur. J. Neurosci._ 48, 3117–3125 (2018). Article PubMed
PubMed Central Google Scholar * Asmussen, M. J., Jacobs, M. F., Lee, K. G. H. H., Zapallow, C. M. & Nelson, A. J. Short-latency afferent inhibition modulation during finger movement.
_PLoS One_ 8, 60496 (2013). Article ADS CAS Google Scholar * Bonassi, G. _et al._ Selective sensorimotor modulation operates during cognitive representation of movement. _Neuroscience_
409, 16–25 (2019). Article CAS PubMed Google Scholar * Fadiga, L., Fogassi, L., Pavesi, G. & Rizzolatti, G. Motor facilitation during action observation: A magnetic stimulation
study. _J. Neurophysiol._ 73, 2608–2611 (1995). Article CAS PubMed Google Scholar * Fadiga, L., Craighero, L. & Olivier, E. Human motor cortex excitability during the perception of
others’ action. _Curr. Opin. Neurobiol._ 15, 213–218 (2005). Article CAS PubMed Google Scholar * Borgomaneri, S., Bolloni, C., Sessa, P. & Avenanti, A. Blocking facial mimicry
affects recognition of facial and body expressions. _PLoS One_ 15, e0229364 (2020). Article CAS PubMed PubMed Central Google Scholar * Oya, H., Kawasaki, H., Howard, M. A. &
Adolphs, R. Electrophysiological responses in the human amygdala discriminate emotion categories of complex visual stimuli. _J. Neurosci._ 22, 9502–9512 (2002). Article CAS PubMed PubMed
Central Google Scholar * Vuilleumier, P., Armony, J. L., Driver, J. & Dolan, R. J. Distinct spatial frequency sensitivities for processing faces and emotional expressions. _Nat.
Neurosci._ 6, 624–631 (2003). Article CAS PubMed Google Scholar * Sagaspe, P., Schwartz, S. & Vuilleumier, P. Fear and stop: A role for the amygdala in motor inhibition by emotional
signals. _Neuroimage_ 55, 1825–1835 (2011). Article PubMed Google Scholar * Avenanti, A., Minio-Paluello, I., Sforza, A. & Aglioti, S. M. Freezing or escaping? Opposite modulations of
empathic reactivity to the pain of others. _Cortex_ 45, 1072–1077 (2009). Article PubMed Google Scholar * Minio-Paluello, I., Baron-Cohen, S., Avenanti, A., Walsh, V. & Aglioti, S.
M. Absence of embodied empathy during pain observation in asperger syndrome. _Biol. Psychiatry_ 65, 55–62 (2009). Article PubMed Google Scholar * Carver, C. S. & White, T. L.
Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS scales. _J. Pers. Soc. Psychol._
https://doi.org/10.1525/curh.2005.104.685.380 (1994). Article PubMed Google Scholar * Leone, L., Pierro, A. & Mannetti, L. Validità della versione italiana delle scale BIS/BAS di
Carver e White (1994): generalizzabilità della struttura e relazioni con costrutti affini. _G. Ital. di Psicol._ 29, 413–436 (2002). Google Scholar * Amodio, D. M., Master, S. L., Yee, C.
M. & Taylor, S. E. Neurocognitive components of the behavioral inhibition and activation systems: Implications for theories of self-regulation. _Psychophysiology_ 45, 11–19 (2008).
PubMed Google Scholar * Kennis, M., Rademaker, A. R. & Geuze, E. Neural correlates of personality: An integrative review. _Neurosci. Biobehav. Rev._ 37, 73–95 (2013). Article PubMed
Google Scholar * Oldfield, R. C. The assessment and analysis of handedness: The Edinburgh inventory. _Neuropsychologia_ 9, 97–113 (1971). Article CAS PubMed Google Scholar * Rossi, S.,
Hallett, M., Rossini, P. M. & Pascual-Leone, A. Screening questionnaire before TMS: An update. _Clin. Neurophysiol._ 122, 1686 (2011). Article PubMed Google Scholar * Association, W.
M. World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. _Bull. World Health Organ._ 79, 373–374 (2001). Google Scholar *
Garaizar, P., Vadillo, M. A., López-De-Ipiña, D. & Matute, H. Measuring software timing errors in the presentation of visual stimuli in cognitive neuroscience experiments. _PLoS One_ 9,
e85108 (2014). Article PubMed PubMed Central ADS CAS Google Scholar * Barke, A., Stahl, J. & Kröner-Herwig, B. Identifying a subset of fear-evoking pictures from the IAPS on the
basis of dimensional and categorical ratings for a German sample. _J. Behav. Ther. Exp. Psychiatry_ 43, 565–572 (2012). Article PubMed Google Scholar * Fischer, M. & Orth, M.
Short-latency sensory afferent inhibition: Conditioning stimulus intensity, recording site, and effects of 1 Hz repetitive TMS. _Brain Stimul._ 4, 202–209 (2011). Article CAS PubMed
Google Scholar * Rothwell, J. _et al._ Magnetic stimulation: Motor evoked potentials. _Int. Fed. Clin. Neurophysiol. Electroencephal. Clin. Neurophysiol. Suppl._ 52, 97–103 (1999). CAS
Google Scholar * Gray, J. A. Brain systems that mediate both emotion and cognition. _Cogn. Emot._ 4, 269–288 (1990). Article Google Scholar * Asmussen, M. J. _et al._ Modulation of
short-latency afferent inhibition depends on digit and task-relevance. _PLoS One_ 9, e104807 (2014). Article PubMed PubMed Central ADS CAS Google Scholar * Curtin, F. & Schulz, P.
Multiple correlations and Bonferroni’s correction. _Biol. Psychiatry_ 44, 775–777 (1998). Article CAS PubMed Google Scholar * Szabó, G. G., Holderith, N., Gulyás, A. I., Freund, T. F.
& Hájos, N. Distinct synaptic properties of perisomatic inhibitory cell types and their different modulation by cholinergic receptor activation in the CA3 region of the mouse
hippocampus. _Eur. J. Neurosci._ 31, 2234–2246 (2010). Article PubMed PubMed Central Google Scholar * Tremblay, N., Warren, R. A. & Dykes, R. W. Electrophysiological studies of
acetylcholine and the role of the basal forebrain in the somatosensory cortex of the cat. I. Cortical neurons excited by glutamate. _J. Neurophysiol._ 64, 1199–1211 (1990). Article CAS
PubMed Google Scholar * Suzuki, L. Y. & Meehan, S. K. Attention focus modulates afferent input to motor cortex during skilled action. _Hum. Mov. Sci._ 74, 102716 (2020). Article CAS
PubMed Google Scholar * Taylor-Clarke, M., Kennett, S. & Haggard, P. Vision modulates somatosensory cortical processing. _Curr. Biol._ 12, 233–236 (2002). Article CAS PubMed Google
Scholar * Bufalari, I., Aprile, T., Avenanti, A., Di Russo, F. & Aglioti, S. M. Empathy for pain and touch in the human somatosensory cortex. _Cereb. Cortex_ 17, 2553–2561 (2007).
Article PubMed Google Scholar * Keysers, C., Kaas, J. H. & Gazzola, V. Somatosensation in social perception. _Nat. Rev. Neurosci._ 11, 417–428 (2010). Article CAS PubMed Google
Scholar * Wood, R., Gallese, V. & Cattaneo, L. Visuotactile empathy within the primary somatosensory cortex revealed by short-latency afferent inhibition. _Neurosci. Lett._ 473, 28–31
(2010). Article CAS PubMed Google Scholar * Lang, P. J., Davis, M. & Öhman, A. Fear and anxiety: Animal models and human cognitive psychophysiology. _J. Affect. Disord._ 61, 137–159
(2000). Article CAS PubMed Google Scholar * Petrovich, G. D., Ross, C. A., Mody, P., Holland, P. C. & Gallagher, M. Central, but not basolateral, amygdala is critical for control of
feeding by aversive learned cues. _J. Neurosci._ 29, 15205–15212 (2009). Article CAS PubMed PubMed Central Google Scholar * Grèzes, J., Valabrègue, R., Gholipour, B. & Chevallier,
C. A direct amygdala-motor pathway for emotional displays to influence action: A diffusion tensor imaging study. _Hum. Brain Mapp._ 35, 5974–5983 (2014). Article PubMed PubMed Central
Google Scholar * Gu, Y. _et al._ A brainstem-central amygdala circuit underlies defensive responses to learned threats. _Mol. Psychiatry_ 25, 640–654 (2020). Article PubMed Google Scholar
* Aston-Jones, G., Iba, M., Clayton, E., Rajkowski, J. & Cohen, J. The locus coeruleus and regulation of behavioral flexibility and attention: Clinical implications. _Brain
Norepinephrine Neurobiol. Ther._ https://doi.org/10.1017/CBO9780511544156.008 (2007). Article Google Scholar * Méndez-Bértolo, C. _et al._ A fast pathway for fear in human amygdala. _Nat.
Neurosci._ 19, 1041–1049 (2016). Article PubMed CAS Google Scholar * Seligman, M. E. P. Phobias and preparedness—Republished article. _Behav. Ther._ 47, 577–584 (2016). Article PubMed
Google Scholar * Johnston, P., Mayes, A., Hughes, M. & Young, A. W. Brain networks subserving the evaluation of static and dynamic facial expressions. _Cortex_ 49, 2462–2472 (2013).
Article PubMed Google Scholar * Meeren, H. K. M., Hadjikhani, N., Ahlfors, S. P., Hämäläinen, M. S. & de Gelder, B. Early category-specific cortical activation revealed by visual
stimulus inversion. _PLoS One_ 3, e3503 (2008). Article PubMed PubMed Central ADS CAS Google Scholar * Barceló, F. & Cooper, P. S. Quantifying contextual information for cognitive
control. _Front. Psychol._ 9, 1693 (2018). Article PubMed PubMed Central Google Scholar * Gontier, E. _et al._ Frontal and parietal ERPs associated with duration discriminations with or
without task interference. _Brain Res._ 1170, 79–89 (2007). Article CAS PubMed Google Scholar * Duncan-Johnson, C. C. & Donchin, E. On quantifying surprise: The variation of
event-related potentials with subjective probability. _Psychophysiology_ 14, 456–467 (1977). Article CAS PubMed Google Scholar * Polich, J. & Kok, A. Cognitive and biological
determinants of P300: An integrative review. _Biol. Psychol._ 41, 103–146 (1995). Article CAS PubMed Google Scholar Download references FUNDING This work was supported by grants from
Italian Ministry of Health assigned to Laura Avanzino (Ricerca Corrente). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Experimental Medicine (DIMES), Section of Human
Physiology, University of Genoa, Viale Benedetto XV/3, 16132, Genoa, Italy Alessandro Botta, Marco Bove & Laura Avanzino * Department of Neuroscience, Rehabilitation, Ophthalmology,
Genetics and Maternal Child Health (DINOGMI), University of Genoa, Genoa, Italy Alessandro Botta, Giovanna Lagravinese & Elisa Pelosin * IRCCS Ospedale Policlinico San Martino, Genoa,
Italy Giovanna Lagravinese, Marco Bove, Elisa Pelosin & Laura Avanzino * S.C. Medicina Fisica e Riabilitazione Ospedaliera, ASL4, Azienda Sanitaria Locale Chiavarese, Chiavari, Italy
Gaia Bonassi * Centro di Neuroscienze Cognitive and Dipartimento di Psicologia, Campus Cesena, Alma Mater Studiorum-University of Bologna, Cesena, Italy Alessio Avenanti * Centro de
Investigación en Neuropsicología y Neurociencias Cognitivas, Universidad Católica del Maule, Talca, Chile Alessio Avenanti Authors * Alessandro Botta View author publications You can also
search for this author inPubMed Google Scholar * Giovanna Lagravinese View author publications You can also search for this author inPubMed Google Scholar * Marco Bove View author
publications You can also search for this author inPubMed Google Scholar * Elisa Pelosin View author publications You can also search for this author inPubMed Google Scholar * Gaia Bonassi
View author publications You can also search for this author inPubMed Google Scholar * Alessio Avenanti View author publications You can also search for this author inPubMed Google Scholar *
Laura Avanzino View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS A.B.: Conceptualization, data curation, investigation, methodology, formal
analysis, supervision, writing—original draft, writing—review and editing. G.L.: Data curation, investigation, methodology, formal analysis, writing—original draft, writing—review and
editing. G.B.: investigation, methodology, writing—review and editing. E.P.: Supervision, writing—review and editing. M.B.: Supervision, writing—review and editing, A.A.: Supervision,
writing—original draft, writing—review and editing. L.A.: Conceptualization, formal analysis, supervision, writing—original draft, writing—review and editing. All authors reviewed the
manuscript. CORRESPONDING AUTHOR Correspondence to Laura Avanzino. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S
NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION. RIGHTS AND
PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any
medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The
images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is
not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission
directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Botta,
A., Lagravinese, G., Bove, M. _et al._ Sensorimotor inhibition during emotional processing. _Sci Rep_ 12, 6998 (2022). https://doi.org/10.1038/s41598-022-10981-8 Download citation *
Received: 10 December 2021 * Accepted: 04 April 2022 * Published: 29 April 2022 * DOI: https://doi.org/10.1038/s41598-022-10981-8 SHARE THIS ARTICLE Anyone you share the following link with
will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative