Genetic variants associated with glaucomatous visual field loss in primary open-angle glaucoma
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Primary open-angle glaucoma (POAG) is characterized by a progressive optic neuropathy with visual field loss. To investigate the genetic variants associated with visual field loss
in POAG, Japanese POAG patients (n = 426) and control subjects (n = 246) were genotyped for 22 genetic variants predisposing to POAG that can be classified into those associated with
intraocular pressure (IOP) elevation (IOP-related genetic variants) and optic nerve vulnerability independent of IOP (optic nerve-related genetic variants). The genetic risk score (GRS) of
the 17 IOP-related and five optic nerve-related genetic variants was calculated, and the associations between the GRS and the mean deviation (MD) of automated static perimetry as an
indicator of the severity of visual field loss and pattern standard deviation (PSD) as an indicator of the focal disturbance were evaluated. There was a significant association (Beta = −
0.51, P = 0.0012) between the IOP-related GRS and MD. The severity of visual field loss may depend on the magnitude of IOP elevation induced by additive effects of IOP-related genetic
variants. A significant association (n = 135, Beta = 0.65, P = 0.0097) was found between the optic nerve-related, but not IOP-related, GRS and PSD. The optic nerve-related (optic nerve
vulnerability) and IOP-related (IOP elevation) genetic variants may play an important role in the focal and diffuse visual field loss respectively. To our knowledge, this is the first report
to show an association between additive effects of genetic variants predisposing to POAG and glaucomatous visual field loss, including severity and focal/diffuse disturbance of visual field
loss, in POAG. SIMILAR CONTENT BEING VIEWED BY OTHERS GWAS-BY-SUBTRACTION REVEALS AN IOP-INDEPENDENT COMPONENT OF PRIMARY OPEN ANGLE GLAUCOMA Article Open access 17 October 2024 MLIP
GENOTYPE AS A PREDICTOR OF PHARMACOLOGICAL RESPONSE IN PRIMARY OPEN-ANGLE GLAUCOMA AND OCULAR HYPERTENSION Article Open access 15 January 2021 EXPLORING THE ROLE OF APOLIPOPROTEIN E GENE
PROMOTER POLYMORPHISMS IN SUSCEPTIBILITY TO NORMAL-TENSION GLAUCOMA IN A KOREAN POPULATION Article Open access 18 April 2024 INTRODUCTION Glaucoma is characterized by a chronic progressive
optic neuropathy with corresponding and characteristic patterns of visual field loss. In most cases, glaucomatous visual field loss is initially localized in the nasal or in the arcuate
region and as the disease progresses, the focal loss becomes wider, deeper, and more numerous. Finally, some cases become blind even while they are receiving therapy. Primary open-angle
glaucoma (POAG) represents the most prevalent form of glaucoma, and clinically, intraocular pressure (IOP) elevation and myopia are reported to be risk factors for optic nerve damage in
POAG1. Additionally, a positive family history of glaucoma is a major risk factor for POAG1,2,3,4,5,6, and genetic factors are therefore considered to play an important role in the
pathogenesis of POAG. Genetic analyses, including genome-wide association study (GWAS), have recently identified genetic variants predisposing to
POAG7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28. These genetic variants can be classified into two types: one type involves genetic variants associated with IOP elevation
(IOP-related genetic variants); the other involves genetic variants associated with vulnerability of the optic nerve, independent of IOP (optic nerve-related genetic variants), which may
include genetic variants associated with apoptosis of optic nerve29, myopia1, and optic nerve circulation30. Moreover, previous studies have reported the additive effects of these genetic
variants on clinical features, such as phenotypes including normal tension glaucoma (NTG) and high tension glaucoma (HTG)19,26,27,31,32,33,34, vertical cup-to-disc ratio (VCDR)35,
IOP23,36,37, family history of glaucoma27,33,37,38, age at diagnosis of glaucoma27,37,38,39, number of medications37, and surgical intervention27,37. However, an association between the
additive effects of genetic variants predisposing to POAG and glaucomatous visual field loss, the most important clinical symptom in POAG, has not been found. In order to further elucidate
the genetic mechanism of visual field loss in POAG, the present study was conducted to investigate the association between the IOP-related/optic nerve-related genetic variants and the mean
deviation (MD) of automated static perimetry as an indicator of the severity of visual field loss and the pattern standard deviation (PSD) as an indicator of the focal visual field loss.
RESULTS Six hundred seventy-two Japanese patients, including 426 patients with POAG (HTG, n = 210; NTG, n = 216) and 246 control subjects, were enrolled in the present study. The demographic
and clinical data for all participants are shown in Table 1. The mean age at the blood sampling was 63.1 ± 13.6 years (standard deviation) in patients with POAG and 67.7 ± 11.2 years in the
control subjects. The mean of maximum IOP was 23.4 ± 7.7 mmHg in patients with POAG and 15.0 ± 2.6 mmHg in the control subjects. ASSOCIATION BETWEEN THE GENETIC RISK SCORE (GRS) AND MD The
mean MD of automated static perimetry (Humphrey Field Analyzer 30–2: HFA30-2, Humphrey Instruments, San Leandro, CA) in the worse eye were -15.1 ± 8.0 dB in patients with POAG. The results
of a multiple linear regression analysis with the MD as a dependent variable and age, sex and the GRS as independent variables are shown in Table 2. There was a significant association (Beta
= − 0.51, 95% confidence interval CI − 0.81 to − 0.20, P = 0.0012) between the GRS of IOP-related genetic variants and the MD. As the GRS of IOP-related genetic variants increased, the MD
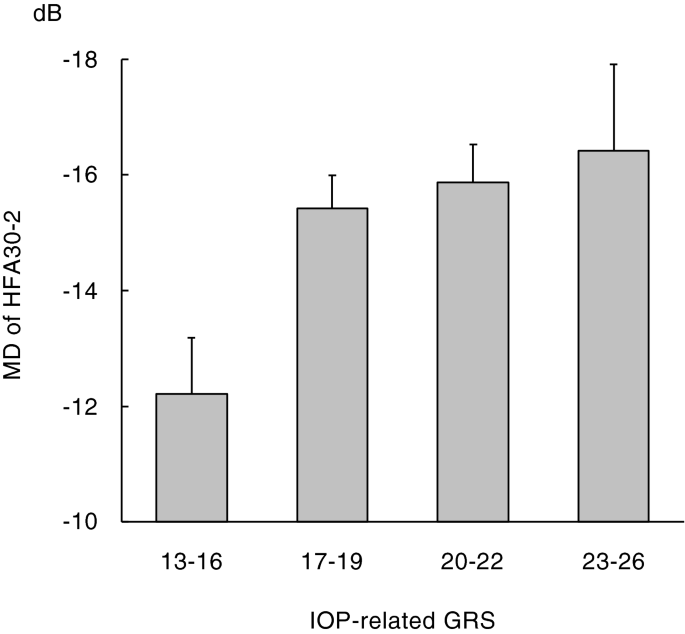
decreased. A graphical representation of mean MD values divided by the GRS of IOP-related genetic variants is shown in Fig. 1. ASSOCIATION BETWEEN THE GRS AND PSD One hundred thirty-five
patients with early to moderate stage POAG were enrolled in this analysis. The mean MD and PSD of HFA30-2 in the worse eye were − 6.9 ± 2.6 and 9.1 ± 3.2 dB respectively. The results of a
multiple linear regression analysis with the PSD as a dependent variable and age, sex and the GRS as independent variables are shown in Table 3. There was a significant association (Beta =
0.65, 95% CI 0.16–1.15, P = 0.0097) between the GRS of optic nerve-related genetic variants and the PSD. As the GRS of optic nerve-related genetic variants increased, the PSD increased. A
graphical representation of mean PSD values divided by the GRS of optic nerve-related genetic variants is shown in Fig. 2. DISCUSSION In the present study, we investigated the association
between the IOP-related/optic nerve-related genetic variants and MD as an indicator of the severity or PSD as an indicator of the focal disturbance of glaucomatous visual field loss in POAG.
There was a significant association between the IOP-related GRS and MD, and as the IOP-related GRS increased, the MD decreased. This result indicates that the severity (MD) of glaucomatous
visual field loss may depend on the magnitude of IOP elevation induced by additive effects of IOP-related genetic variants. It may be reasonable, as it is clinically reported that reducing
the IOP in glaucomatous eyes prevents disease progression. The IOP-related GRS is also reported to be associated with age at the diagnosis of glaucoma as an indicator of the progression of
POAG27,38, which may support the results of the present study. No significant association has previously been found between the additive effects of IOP-related genetic variants and MD37. The
POAG patients with a wider range of maximum IOP (IOP-related GRS) might be included in the present study because the prevalence of NTG in the Japanese population is higher than that in
other ethnic populations40. This may be the reason why a significant association could be found between them in the present study. Previous studies have reported the association between the
genetic variants near _CAV1/CAV2_41 or _p53_42 and POAG with paracentral visual field loss. In the present study, a significant association was found between the optic nerve-related GRS and
PSD. As the optic nerve-related GRS increased, the PSD increased. This result indicates that the additive effects of optic nerve-related genetic variants are associated with focal
glaucomatous visual field loss. It has been reported that focal glaucomatous visual field loss occurs at a lower IOP than diffuse loss and—as such—may be a marker that can be used to
identify patients whose optic nerves are abnormally susceptible to glaucomatous injury43. Focal glaucomatous visual field loss may occur due to vulnerability of the optic nerve induced by
additive effects of optic nerve-related genetic variants. In other words, the optic nerve vulnerability induced by optic nerve-related genetic variants may result in typical glaucomatous
visual field loss, such as nasal step and/or partial arcuate visual field loss. In contrast, as described above, the IOP-related GRS was associated with the MD, but not the PSD, which gives
an overall value of the total amount of visual function loss, but not the localized visual function loss. A previous study reported that POAG patients with diffuse visual field depression
manifested higher IOP than those with localized visual field defects44. It was also reported that IOP was significantly higher in patients with generalized enlargement of the optic cup
discs, which indicates diffuse glaucomatous visual field loss45. These results indicate that IOP-related genetic variants are associated with diffuse glaucomatous visual field loss. On the
whole, the optic nerve-related (optic nerve vulnerability) and IOP-related (IOP elevation) genetic variants may contribute to focal and diffuse glaucomatous visual field loss respectively.
To our knowledge, this is the first report to show an association between additive effects of genetic variants predisposing to POAG and glaucomatous visual field loss, including severity and
focal/diffuse disturbance of visual field loss, in POAG. To evaluate the additive effects of genetic variants predisposing to POAG, the total number of risk alleles of multi-locus genetic
variants was used as an unweighted GRS in the present study. Given that the unweighted GRS approach assumed that all risk alleles had the same magnitude of effect on the risk of POAG, the
results might not precisely reflect the additive effects of the genetic variants. Thus, in a previous study that reported the additive effects of genetic variants on the risk of POAG34, a
logistic regression model was used to estimate the risk (odds ratio) of glaucoma for each risk allele of the genetic variants, and the sum of the logarithmically-converted odds ratios of
multi-locus genetic variants was used as a weighted GRS. In the present study, the results obtained using this weighted GRS approach (Supplementary Tables 1, 2) were fundamentally the same
as those obtained using the unweighted GRS approach. With regard to limitations, some genetic variants16,17,18,19,20,21,22,23,24,25,26,27,28,35 that have been reported to be associated with
susceptibility to POAG were not analyzed in the present study. An analysis that includes these genetic variants may be better for evaluating the complex genetic mechanism of POAG, although
all reported IOP-related genetic variants should not be included to reduce contamination of optic nerve-related genetic variants in IOP-related genetic variants. GRS studies incorporating
additional genetic variants are an important future direction. Media opacity, such as a cataract, has been shown to affect the results of automated static perimetry46, and some patients with
cataract were included in the present study. To reduce the influence of cataract on the MD and PSD, POAG patients with a best corrected visual acuity of > 20/25 were selected and
analyzed. The results obtained using these selected subjects were fundamentally the same as those obtained using the unselected original subjects: the associations between the IOP-related
GRS and MD (n = 292, Beta = − 0.36, 95% CI − 0.67 to − 0.040, P = 0.027), optic nerve-related GRS and PSD (n = 117, Beta = 0.81, 95% CI 0.29–1.33, P = 0.0026). The participants of the
present study were all Japanese. Since the genetic background differs between ethnicities, further studies may be necessary to generalize our findings to other ethnic populations. In
summary, glaucomatous visual field loss in cases of POAG is influenced by the genetic variants predisposing to POAG. The severity (MD) of visual field loss was associated with additive
effects of IOP-related genetic variants, and therefore accounts for the role of IOP elevation as a risk factor for POAG. The optic nerve-related genetic variants were associated with PSD as
an indicator of the focal visual field loss, while the IOP-related genetic variants were not. These results indicate that optic nerve vulnerability to IOP due to optic nerve-related genetic
variants may play an important role in the focal visual field loss and that IOP elevation induced by IOP-related genetic variants may play an important role in the diffuse visual field loss
in POAG. The present findings are useful for understanding the pathogenesis of glaucomatous visual field loss in POAG. METHODS SUBJECTS Japanese patients with POAG were recruited from the
ophthalmology practices at the Enzan Municipal Hospital, Oizumi Clinic, Uenohara City Hospital, and Yamanashi University Hospital in Yamanashi Prefectures, Japan. POAG was diagnosed when an
open anterior chamber angle was detected on a gonioscopic examination, and the typical glaucomatous changes in the optic nerve head (enlargement of the VCDR, and/or focal notching of the
optic disc rim, and/or retinal nerve fiber layer defect resulting in a thinning in the neuroretinal rim) with a compatible visual field loss (nasal step and/or partial arcuate visual field
loss, etc.) was observed in at least one eye. Anderson-Patella’s criteria47 were used to define glaucomatous visual field loss. Briefly, the criteria were as follows: a cluster of ≥ 3 points
in the pattern deviation plot in a single hemifield (superior/inferior) with P < 0.05, one of which had to have been P < 0.01, on HFA30-2. In addition, patients were diagnosed with
HTG when they had at least one previous IOP measurement of ≥ 22 mmHg with a Goldmann applanation tonometer. Patients with NTG showed an IOP of ≤ 21 mmHg each time they were tested. The
highest IOP in both eyes, chosen from all of the measured IOPs in the patient’s medical records was considered to be the maximum IOP, and IOPs measured after surgical treatments were
excluded. Patients who had a history of eye surgery, including laser treatment, before the diagnosis of POAG were excluded from the present study. The control subjects, who were recruited
from participating institutions to estimate the risk (odds ratio) of glaucoma for each risk allele of genetic variants predisposing to POAG and to calculate a weighted GRS, included Japanese
individuals who were over 40 years of age, with an IOP of ≤ 21 mmHg, who exhibited no glaucomatous cupping of the optic disc (no thinning of disc rim and VCDR ≤ 0.4), and who had no family
history of glaucoma. Comprehensive ophthalmologic examinations including both slit-lamp biomicroscopy and fundoscopy were performed and written informed consent was obtained from all study
participants. The study protocol was prospectively approved by the Ethics Committee of University of Yamanashi, and the present study was conducted in accordance with the Declaration of
Helsinki. EVALUATION OF GLAUCOMATOUS VISUAL FIELD LOSS The mean deviation (MD) and pattern standard deviation (PSD) of HFA30-2 in the worse eye were used to evaluate glaucomatous visual
field loss in the present study. Eyes with unreliable visual field results defined as > 30% false-negative results, > 30% false-positive results, or > 20% fixation losses were
excluded. Eyes with neurological or ocular diseases that could cause visual field loss were also excluded. The number of visual field tests depends on the case, and the latest results within
the reliable visual field tests were used for analyses. The MD is an useful indicator that shows a linear change with the progression of glaucoma and was used to evaluate the severity of
glaucomatous visual field loss. Blumenthal and associates48 reported that the MD value of eyes that are unable to perform automated static perimetry due to poor vision levels corresponds to
the value of − 31.43 dB. The MD values of seven eyes that were unable to perform visual field test due to poor vision levels, such as light perception, were assigned values of − 31.43 dB.
The PSD is an useful indicator of localized functional loss and was used to evaluate the focal glaucomatous visual field loss. The PSD is based on the pattern deviation plot, and as the
visual field loss becomes more diffuse, their values return to normal (toward zero). In fact, the correlations between the MD and PSD values are not linear. Higher PSD values are found with
increasing visual field loss, as determined by MD. However, this initial trend is reversed with further functional loss (eyes with MD < − 17 dB approximately)49. Thus, PSD is not a good
parameter to monitor eyes with advanced POAG. Eyes with early to moderate stage POAG (− 10.99 ≤ MD ≤ − 1.77 dB) were selected to evaluate the association between the PSD and
IOP-related/optic nerve-related genetic variants. GENOMIC DNA GENOTYPING Genomic DNA was purified from peripheral blood with a Flexi Gene® DNA Kit (QIAGEN, Valencia, CA, USA). There are 22
genetic variants that predispose individuals to POAG—17 variants identified as IOP-related genetic variants on GWAS, including rs1052990 (near gene: _CAV2_)8,50, rs11656696 (_GAS7_)51,
rs59072263 (_GLCCI1/ICA1_)52, rs2472493 (_ABCA1_)8,9,10, rs58073046 (_ARHGEF12_)14, rs2286885 (_FAM125B/LMX1B_)12,18, rs8176743 (_ABO_)8, rs747782 (_PTPRJ_)8, rs4619890 (_AFAP1_)9,
rs11969985 _(GMDS_)9, rs2745572 (_FOXC1_)15, rs35934224 (_TXNRD2_)15, rs6732795 (_ANTXR1_)18, rs9853115 (_DGKG_)23, rs10505100 _(ANGPT1_)23, rs7924522 (_ETS1_)23 and rs61394862 (_ANKH_)23
and 5 variants considered to be optic nerve-related genetic variants, including rs3213787 (_SRBD1_)53, rs735860 (_ELOVL5_)53, rs1063192 (_CDKN2B_)54, rs10483727 (_SIX6_)54, and rs61861119
(_MYOF_)23, were genotyped using TaqMan single nucleotide polymorphism genotyping assays (Applied Biosystems [ABI], Foster City, CA, USA). Assays were performed on a 7300/7500 Real-Time PCR
System (ABI, Foster City, CA, USA) according to the manufacturer’s instructions. The frequency of patients with optic nerve-related genetic variants is high in patients with POAG. Similarly,
the frequency of patients with high IOP is also high in patients with POAG. The possibility can’t be completely denied that statistically significant association between the optic
nerve-related genetic variants and IOP had been found by the confounding effect on GWAS, especially one with higher statistical power by large number of samples, and that optic nerve-related
genetic variants had been identified as IOP-related genetic variants. To reduce contamination of optic nerve-related genetic variants in IOP-related genetic variants, the genotyped genetic
variants were selected as previously described38. Briefly, in addition to the IOP-related genetic variants reported before 2017, the IOP-related genetic variants with top 10 statistically
significant association with IOP reported by MacGregor and associates23 in 2018 were selected and included in the present study. The IOP-related genetic variants associated with corneal
thickness, such as variants near _FNDC3B_8,55 and _ADAMTS8_16,55, were excluded. The IOP-related genetic variants near _TMCO1_56 and _ATXN2_15 were not included because these variants were
not polymorphic or rare in the Japanese population. The genetic variants near _SRBD1_ and _ELOVL5_ were included as optic nerve-related variants in the present study because these variants
were identified in 2010 on GWAS of Japanese patients with early-onset NTG53, in which the IOPs are consistently within the statistically normal range for the general population. The genetic
variants near _CDKN2B_, _SIX6_, and _MYOF_ were also selected as optic nerve-related variants because these variants were reported to be associated with POAG but not IOP by MacGregor and
associates23 in 2018 when the present study was conducted. STATISTICAL ANALYSIS Data analysis was performed using JMP statistical software version 14.3.0 (SAS Institute Inc., Cary, NC, USA).
The demographic and clinical data in patients with POAG and control subjects were compared using Fisher exact test for comparison of proportion and Student t test for continuous variables.
To evaluate the additive effects of IOP-related and optic nerve-related genetic variants, the total number of risk alleles of the 17 IOP-related (range: 0–34) and 5 optic nerve-related
(range: 0–10) genetic variants were calculated for each participant as a genetic risk score (GRS). To elucidate the genetic variants associated with glaucomatous visual field loss, the
associations between the GRS and MD (as an indicator of the severity of visual field loss) or PSD (as an indicator of the focal disturbance of visual field loss) were evaluated using a
multiple linear regression analysis adjusted for age and sex. A value of P < 0.05 was considered to be statistically significant. DATA AVAILABILITY The dataset generated during and/or
analyzed during the present study is available in the figshare repository, https://figshare.com/s/74882d717c7a717ee5c6. REFERENCES * Hollands, H. _et al._ Do findings on routine examination
identify patients at risk for primary open-angle glaucoma? The rational clinical examination systematic review. _JAMA_ 309, 2035–2042 (2013). Article CAS PubMed Google Scholar * Tielsch,
J. M., Katz, J., Sommer, A., Quigley, H. A. & Javitt, J. C. Family history and risk of primary open angle glaucoma. The Baltimore Eye survey. _Arch. Ophthalmol._ 112, 69–73 (1994).
Article CAS PubMed Google Scholar * Leske, M. C., Connell, A. M., Wu, S. Y., Hyman, L. G. & Schachat, A. P. Risk factors for open-angle glaucoma. The Barbados Eye study. _Arch.
Ophthalmol._ 113, 918–924 (1995). Article CAS PubMed Google Scholar * Wolfs, R. C. _et al._ Genetic risk of primary open-angle glaucoma. Population-based familial aggregation study.
_Arch. Ophthalmol._ 116, 1640–1645 (1998). Article CAS PubMed Google Scholar * Weih, L. M., Nanjan, M., McCarty, C. A. & Taylor, H. R. Prevalence and predictors of open-angle
glaucoma: Results from the visual impairment project. _Ophthalmology_ 108, 1966–1972 (2001). Article CAS PubMed Google Scholar * Sun, J. _et al._ Prevalence and risk factors for primary
open-angle glaucoma in a rural northeast China population: A population-based survey in Bin County Harbin. _Eye_ 26, 283–291 (2012). Article CAS PubMed Google Scholar * Janssen, S. F.
_et al._ The vast complexity of primary open angle glaucoma: Disease genes, risks, molecular mechanisms and pathobiology. _Prog. Retin. Eye Res._ 37, 31–67 (2013). Article CAS PubMed
Google Scholar * Hysi, P. G. _et al._ Genome-wide analysis of multi-ancestry cohorts identifies new loci influencing intraocular pressure and susceptibility to glaucoma. _Nat. Genet._ 46,
1126–1130 (2014). Article CAS PubMed PubMed Central Google Scholar * Gharahkhani, P. _et al._ Common variants near _ABCA1_, _AFAP1_ and _GMDS_ confer risk of primary open-angle
glaucoma. _Nat. Genet._ 46, 1120–1125 (2014). Article CAS PubMed PubMed Central Google Scholar * Chen, Y. _et al._ Common variants near _ABCA1_ and in _PMM2_ are associated with primary
open-angle glaucoma. _Nat. Genet._ 46, 1115–1119 (2014). Article CAS PubMed Google Scholar * Springelkamp, H. _et al._ Meta-analysis of genome-wide association studies identifies novel
loci that influence cupping and the glaucomatous process. _Nat. Commun._ 5, 4883 (2014). Article CAS PubMed Google Scholar * Nag, A. _et al._ A genome-wide association study of
intra-ocular pressure suggests a novel association in the gene _FAM125B_ in the TwinsUK cohort. _Hum. Mol. Genet._ 23, 3343–3348 (2014). Article CAS PubMed PubMed Central Google Scholar
* Li, Z. _et al._ A common variant near _TGFBR3_ is associated with primary open angle glaucoma. _Hum. Mol. Genet._ 24, 3880–3892 (2015). Article CAS PubMed PubMed Central Google
Scholar * Springelkamp, H. _et al._ _ARHGEF12_ influences the risk of glaucoma by increasing intraocular pressure. _Hum. Mol. Genet._ 24, 2689–2699 (2015). Article CAS PubMed Google
Scholar * Bailey, J. N. _et al._ Genome-wide association analysis identifies _TXNRD2_, _ATXN2_ and _FOXC1_ as susceptibility loci for primary open-angle glaucoma. _Nat. Genet._ 48, 189–194
(2016). Article PubMed Google Scholar * Springelkamp, H. _et al._ New insights into the genetics of primary open-angle glaucoma based on meta-analyses of intraocular pressure and optic
disc characteristics. _Hum. Mol. Genet._ 26, 438–453 (2017). CAS PubMed PubMed Central Google Scholar * Chintalapudi, S. R. _et al._ Systems genetics identifies a role for Cacna2d1
regulation in elevated intraocular pressure and glaucoma susceptibility. _Nat. Commun._ 8, 1755 (2017). Article PubMed PubMed Central Google Scholar * Choquet, H. _et al._ A large
multi-ethnic genome-wide association study identifies novel genetic loci for intraocular pressure. _Nat. Commun._ 8, 2108 (2017). Article PubMed PubMed Central Google Scholar *
Bonnemaijer, P. W. M. _et al._ Genome-wide association study of primary open-angle glaucoma in continental and admixed African populations. _Hum. Genet._ 137, 847–862 (2018). Article CAS
PubMed PubMed Central Google Scholar * Gao, X. R., Huang, H., Nannini, D. R., Fan, F. & Kim, H. Genome-wide association analyses identify new loci influencing intraocular pressure.
_Hum. Mol. Genet._ 27, 2205–2213 (2018). Article CAS PubMed PubMed Central Google Scholar * Gharahkhani, P. _et al._ Analysis combining correlated glaucoma traits identifies five new
risk loci for open-angle glaucoma. _Sci. Rep._ 8, 3124 (2018). Article PubMed PubMed Central Google Scholar * Khawaja, A. P. _et al._ Genome-wide analyses identify 68 new loci associated
with intraocular pressure and improve risk prediction for primary open-angle glaucoma. _Nat. Genet._ 50, 778–782 (2018). Article CAS PubMed PubMed Central Google Scholar * MacGregor,
S. _et al._ Genome-wide association study of intraocular pressure uncovers new pathways to glaucoma. _Nat. Genet._ 50, 1067–1071 (2018). Article CAS PubMed Google Scholar * Shiga, Y. _et
al._ Genome-wide association study identifies seven novel susceptibility loci for primary open-angle glaucoma. _Hum. Mol. Genet._ 27, 1486–1496 (2018). Article CAS PubMed PubMed Central
Google Scholar * Genetics of Glaucoma in People of African Descent C _et al._ Association of genetic variants with primary open-angle glaucoma among individuals with African ancestry.
_JAMA_ 322, 1682–1691 (2019). Article Google Scholar * Taylor, K. D. _et al._ Genetic architecture of primary open-angle glaucoma in individuals of African descent: The African descent and
glaucoma evaluation study III. _Ophthalmology_ 126, 38–48 (2019). Article PubMed Google Scholar * Craig, J. E. _et al._ Multitrait analysis of glaucoma identifies new risk loci and
enables polygenic prediction of disease susceptibility and progression. _Nat. Genet._ 52, 160–166 (2020). Article CAS PubMed PubMed Central Google Scholar * Gharahkhani, P. _et al._
Genome-wide meta-analysis identifies 127 open-angle glaucoma loci with consistent effect across ancestries. _Nat. Commun._ 12, 1258 (2021). Article CAS PubMed PubMed Central Google
Scholar * Almasieh, M., Wilson, A. M., Morquette, B., Cueva Vargas, J. L. & Di Polo, A. The molecular basis of retinal ganglion cell death in glaucoma. _Prog. Retin. Eye Res._ 31,
152–181 (2012). Article CAS PubMed Google Scholar * Flammer, J. & Orgul, S. Optic nerve blood-flow abnormalities in glaucoma. _Prog. Retin. Eye Res._ 17, 267–289 (1998). Article CAS
PubMed Google Scholar * Ramdas, W. D. _et al._ Clinical implications of old and new genes for open-angle glaucoma. _Ophthalmology_ 118, 2389–2397 (2011). Article PubMed Google Scholar
* Ramdas, W. D. _et al._ Genetic architecture of open angle glaucoma and related determinants. _J. Med. Genet._ 48, 190–196 (2011). Article PubMed Google Scholar * Mabuchi, F. _et al._
Involvement of genetic variants associated with primary open-angle glaucoma in pathogenic mechanisms and family history of glaucoma. _Am. J. Ophthalmol._ 159, 437–44 e2 (2015). Article CAS
PubMed Google Scholar * Tham, Y. C. _et al._ Aggregate effects of intraocular pressure and cup-to-disc ratio genetic variants on glaucoma in a multiethnic Asian population.
_Ophthalmology_ 122, 1149–1157 (2015). Article PubMed Google Scholar * Nannini, D. R., Kim, H., Fan, F. & Gao, X. Genetic risk score is associated with vertical cup-to-disc ratio and
improves prediction of primary open-angle glaucoma in Latinos. _Ophthalmology_ 125, 815–821 (2018). Article PubMed Google Scholar * Mabuchi, F. _et al._ Additive effects of genetic
variants associated with intraocular pressure in primary open-angle glaucoma. _PLoS ONE_ 12, e0183709 (2017). Article PubMed PubMed Central Google Scholar * Qassim, A. _et al._ An
intraocular pressure polygenic risk score stratifies multiple primary open-angle glaucoma parameters including treatment intensity. _Ophthalmology_ 127, 901–907 (2020). Article PubMed
Google Scholar * Mabuchi, F. _et al._ Genetic variants associated with the onset and progression of primary open-angle glaucoma. _Am. J. Ophthalmol._ 215, 135–140 (2020). Article CAS
PubMed Google Scholar * Fan, B. J. _et al._ Association of a primary open-angle glaucoma genetic risk score with earlier age at diagnosis. _JAMA Ophthalmol._ 137, 1190–1194 (2019). Article
PubMed PubMed Central Google Scholar * Iwase, A. _et al._ The prevalence of primary open-angle glaucoma in Japanese: The Tajimi study. _Ophthalmology_ 111, 1641–1648 (2004). PubMed
Google Scholar * Loomis, S. J. _et al._ Association of _CAV1/CAV2_ genomic variants with primary open-angle glaucoma overall and by gender and pattern of visual field loss. _Ophthalmology_
121, 508–516 (2014). Article PubMed Google Scholar * Wiggs, J. L. _et al._ The _p53_ codon 72 PRO/PRO genotype may be associated with initial central visual field defects in caucasians
with primary open angle glaucoma. _PLoS ONE_ 7, e45613 (2012). Article CAS PubMed PubMed Central Google Scholar * Samuelson, T. W. & Spaeth, G. L. Focal and diffuse visual field
defects: Their relationship to intraocular pressure. _Ophthalmic Surg._ 24, 519–525 (1993). CAS PubMed Google Scholar * Caprioli, J., Sears, M. & Miller, J. M. Patterns of early
visual field loss in open-angle glaucoma. _Am. J. Ophthalmol._ 103, 512–517 (1987). Article CAS PubMed Google Scholar * Nicolela, M. T. & Drance, S. M. Various glaucomatous optic
nerve appearances: Clinical correlations. _Ophthalmology_ 103, 640–649 (1996). Article CAS PubMed Google Scholar * Koucheki, B., Nouri-Mahdavi, K., Patel, G., Gaasterland, D. &
Caprioli, J. Visual field changes after cataract extraction: The AGIS experience. _Am. J. Ophthalmol._ 138, 1022–1028 (2004). Article PubMed Google Scholar * Anderson, D. R. &
Patella, V. M. _Automated Static Perimetry_ 121–136 (Mosby St. Louis, 1999). Google Scholar * Blumenthal, E. Z. & Sapir-Pichhadze, R. Misleading statistical calculations in far-advanced
glaucomatous visual field loss. _Ophthalmology_ 110, 196–200 (2003). Article PubMed Google Scholar * Sousa, M. C. _et al._ Suitability of the visual field index according to glaucoma
severity. _J. Curr. Glaucoma Pract._ 9, 65–68 (2015). Article PubMed Google Scholar * Thorleifsson, G. _et al._ Common variants near _CAV1_ and _CAV2_ are associated with primary
open-angle glaucoma. _Nat. Genet._ 42, 906–909 (2010). Article CAS PubMed PubMed Central Google Scholar * van Koolwijk, L. M. _et al._ Common genetic determinants of intraocular
pressure and primary open-angle glaucoma. _PLoS Genet._ 8, e1002611 (2012). Article PubMed PubMed Central Google Scholar * Blue Mountains Eye Study & Wellcome Trust Case Control
Consortium 2. Genome-wide association study of intraocular pressure identifies the _GLCCI1/ICA1_ region as a glaucoma susceptibility locus. _Hum. Mol. Genet._ 22, 4653–60 (2013). Article
Google Scholar * Writing Committee for the Normal Tension Glaucoma Genetic Study Group of Japan Glaucoma Society _et al._ Genome-wide association study of normal tension glaucoma: Common
variants in _SRBD1_ and _ELOVL5_ contribute to disease susceptibility. _Ophthalmology_ 117, 1331–8 e5 (2010). Article Google Scholar * Ramdas, W. D. _et al._ Common genetic variants
associated with open-angle glaucoma. _Hum. Mol. Genet._ 20, 2464–2471 (2011). Article CAS PubMed Google Scholar * Iglesias, A. I. _et al._ Cross-ancestry genome-wide association analysis
of corneal thickness strengthens link between complex and Mendelian eye diseases. _Nat. Commun._ 9, 1864 (2018). Article PubMed PubMed Central Google Scholar * Burdon, K. P. _et al._
Genome-wide association study identifies susceptibility loci for open angle glaucoma at _TMCO1_ and _CDKN2B-AS1_. _Nat. Genet._ 43, 574–578 (2011). Article CAS PubMed Google Scholar
Download references ACKNOWLEDGEMENTS The authors thank the Japan Glaucoma Society Omics Group (JGS-OG) for in-depth discussions. This study was supported in part by Japan Society for the
Promotion of Science (JSPS) KAKENHI Grant Number 15K10861 and 18K09400. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Ophthalmology, Faculty of Medicine, University of
Yamanashi, Chuo, Yamanashi, Japan Fumihiko Mabuchi, Nakako Mabuchi, Yoichi Sakurada, Seigo Yoneyama & Kenji Kashiwagi * Department of Health Sciences, Faculty of Medicine, University of
Yamanashi, Chuo, Yamanashi, Japan Zentaro Yamagata * Department of Ophthalmology, Saitama Red Cross Hospital, Chuo-ku, Saitama, Japan Mitsuko Takamoto * Department of Ophthalmology, Graduate
School of Medicine, University of Tokyo, Bunkyo-ku, Tokyo, Japan Makoto Aihara * Division of Molecular and Cellular Biology, National Institute of Sensory Organs, National Hospital
Organization Tokyo Medical Center, Meguro-ku, Tokyo, Japan Takeshi Iwata * Department of Ophthalmology, Tohoku University Graduate School of Medicine, Sendai, Miyagi, Japan Kazuki Hashimoto,
Yukihiro Shiga & Toru Nakazawa * Department of Ophthalmic Imaging and Information Analytics, Tohoku University Graduate School of Medicine, Sendai, Miyagi, Japan Kota Sato & Toru
Nakazawa * Collaborative Program for Ophthalmic Drug Discovery, Tohoku University Graduate School of Medicine, Sendai, Miyagi, Japan Toru Nakazawa * Department of Ocular Pathology and
Imaging Science, Graduate School of Medical Sciences, Kyushu University, Fukuoka City, Fukuoka, Japan Masato Akiyama * Yasuma Eye Clinic, Nagoya, Aichi, Japan Kazuhide Kawase * Department of
Ophthalmology Protective Care for Sensory Disorders, Nagoya University Graduate School of Medicine, Nagoya, Aichi, Japan Kazuhide Kawase * Ozaki Eye Hospital, Hyuga, Miyazaki, Japan Mineo
Ozaki * Kanto Central Hospital of the Mutual Aid Association of Public School Teachers, Setagaya-ku, Tokyo, Japan Makoto Araie Authors * Fumihiko Mabuchi View author publications You can
also search for this author inPubMed Google Scholar * Nakako Mabuchi View author publications You can also search for this author inPubMed Google Scholar * Yoichi Sakurada View author
publications You can also search for this author inPubMed Google Scholar * Seigo Yoneyama View author publications You can also search for this author inPubMed Google Scholar * Kenji
Kashiwagi View author publications You can also search for this author inPubMed Google Scholar * Zentaro Yamagata View author publications You can also search for this author inPubMed Google
Scholar * Mitsuko Takamoto View author publications You can also search for this author inPubMed Google Scholar * Makoto Aihara View author publications You can also search for this author
inPubMed Google Scholar * Takeshi Iwata View author publications You can also search for this author inPubMed Google Scholar * Kazuki Hashimoto View author publications You can also search
for this author inPubMed Google Scholar * Kota Sato View author publications You can also search for this author inPubMed Google Scholar * Yukihiro Shiga View author publications You can
also search for this author inPubMed Google Scholar * Toru Nakazawa View author publications You can also search for this author inPubMed Google Scholar * Masato Akiyama View author
publications You can also search for this author inPubMed Google Scholar * Kazuhide Kawase View author publications You can also search for this author inPubMed Google Scholar * Mineo Ozaki
View author publications You can also search for this author inPubMed Google Scholar * Makoto Araie View author publications You can also search for this author inPubMed Google Scholar
CONTRIBUTIONS Concept and design: F.M., K.K. Acquisition or analysis of data: F.M., N.M., Y.S., S.Y., M.T. Interpretation of data: F.M., Y.S., S.Y., K.K., Z.Y., M.T., M.A., T.I., K.H., K.S.,
Y.S., T.N., M.A., K.K., M.O., M.A. Statistical analysis: F.M., Z.Y., M.A. Obtained funding: F.M. Drafting of the manuscript: F.M. Revision of the manuscript: N.M., Y.S., S.Y., K.K., Z.Y.,
M.T., M.A., T.I., K.H., K.S., Y.S., T.N., M.A., K.K., M.O., M.A. CORRESPONDING AUTHOR Correspondence to Fumihiko Mabuchi. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no
competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
SUPPLEMENTARY INFORMATION SUPPLEMENTARY TABLES. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use,
sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative
Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated
otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or
exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints
and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Mabuchi, F., Mabuchi, N., Sakurada, Y. _et al._ Genetic variants associated with glaucomatous visual field loss in primary open-angle
glaucoma. _Sci Rep_ 12, 20744 (2022). https://doi.org/10.1038/s41598-022-24915-x Download citation * Received: 24 February 2022 * Accepted: 22 November 2022 * Published: 01 December 2022 *
DOI: https://doi.org/10.1038/s41598-022-24915-x SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is
not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative