Development and validation of a prognostic nomogram for predicting in-hospital mortality of patients with acute paraquat poisoning
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT This study aimed to develop and validate a predictive model to determine the risk of in-hospital mortality in patients with acute paraquat poisoning. This retrospective
observational cohort study included 724 patients with acute paraquat poisoning whose clinical data were collected within 24 h of admission. The primary outcome was in-hospital mortality.
Patients were randomly divided into training and validation cohorts (7/3 ratio). In the training cohort, the least absolute shrinkage and selection operator regression models were used for
data dimension reduction and feature selection. Multivariate logistic regression was used to generate a predictive nomogram for in-hospital mortality. The prediction model was assessed for
both the training and validation cohorts. In the training cohort, decreased level of consciousness (Glasgow Coma Scale score < 15), neutrophil-to-lymphocyte ratio, alanine
aminotransferase, creatinine, carbon dioxide combining power, and paraquat plasma concentrations at admission were identified as independent predictors of in-hospital mortality in patients
with acute paraquat poisoning. The calibration curves, decision curve analysis, and clinical impact curves indicated that the model had a good predictive performance. It can be used on
admission to the emergency department to predict mortality and facilitate early risk stratification and actionable measures in clinical practice after further external validation. SIMILAR
CONTENT BEING VIEWED BY OTHERS DEVELOPMENT AND VALIDATION OF A PROGNOSTIC NOMOGRAM FOR PREDICTING OF PATIENTS WITH ACUTE SEDATIVE-HYPNOTIC OVERDOSE ADMITTED TO THE INTENSIVE CARE UNIT
Article Open access 27 January 2025 A NOVEL SIMPLE RISK MODEL TO PREDICT THE PROGNOSIS OF PATIENTS WITH PARAQUAT POISONING Article Open access 08 January 2021 A NOMOGRAM FOR PREDICTING EARLY
MORTALITY IN PATIENTS WITH TRAUMATIC BRAIN INJURY REQUIRING MECHANICAL VENTILATION BASED ON CLINICAL LABORATORY DATA Article Open access 26 November 2024 INTRODUCTION Paraquat
(1,1′-dimethyl-4,4′-bipyridinium dichloride [PQ]) is a non-selective contact herbicide widely used in many countries since the 1960s1. Acute PQ poisoning (APP) has been associated with
suicides and accidents, with PQ causing sequential organ damage/failure through oxidative stress and systemic inflammatory responses. The most prominent manifestations include acute lung and
kidney injuries2. The mortality rate of patients with APP remains tremendously high (50–90%)3,4,5,6. Consequently, PQ has been banned in most countries, including China. However, PQ is
still legally used in some areas7,8. Early assessment of the severity and prognosis of patients with APP is crucial to guide treatment (hemodilution, immunosuppressive therapy)9,10.
Currently, no standardized method exists for predicting the prognosis of APP. Previous studies have demonstrated the utility of the Severity Index of PQ Poisoning (SIPP) as a specific
scoring system for prognosticating APP11. However, calculating the SIPP score necessitates the measurement of serum PQ concentration, which demands costly equipment. Consequently, its usage
is limited in most countries, particularly in developing nations12,13. Furthermore, research indicates that the SIPP score might overestimate the survival rate of patients with APP14,
especially those admitted to the hospital more than 24 h after poisoning15. Prior research has identified complete blood cell count16, liver and kidney function indicators17, serum anion
gap18,19, and chest computed tomography20 as independent prognostic indicators for APP. Nevertheless, these indicators are relatively individualistic and demonstrate limited predictive
power. The currently available scoring systems for predicting patient prognosis, including the Acute Physiology and Chronic Health Evaluation II (APACHE II) score21, Sequential Organ Failure
Assessment (SOFA) score22, and Poisoning Severity Score (PSS)23, are designed for critically ill patients rather than individuals with low exposure or mild symptoms. Furthermore, due to the
intricate calculation process, these scoring systems are incapable of promptly predicting the mortality rate or conducting risk assessments for PQ poisoning patients24. Accordingly, it is
imperative to develop an efficient, straightforward, and universally applicable predictive model based on commonly employed laboratory indicators. A nomogram is a simple multivariate
prediction model that incorporates multiple variables affecting prognosis to calculate an individual’s survival probability25. Therefore, this study aims to develop a nomogram model based on
clinical data collected within 24 h of admission that can identify, at an early stage, patients who are at high risk of in-hospital mortality due to APP. METHODS SETTING We performed a
retrospective, observational cohort study in an emergency department (ED) with more than 3000 beds in an extensive tertiary care teaching hospital in Chengdu City, Sichuan Province, China,
per the amended Declaration of Helsinki. The West China Hospital approved the study of the Sichuan University Biomedical Research Ethics Committee (No. 2022-1591). Due to the retrospective
nature of the study, the need of informed consent was waived by the Sichuan University Biomedical Research Ethics Committee. All analyses were performed following the Transparent Reporting
of a multivariate prediction model for Individual Prognosis or Diagnosis statement26. PATIENTS We conducted a study involving patients diagnosed with APP who were admitted to the ED of West
China Hospital, Sichuan University, between September 1, 2010, and January 31, 2022. Inclusion criteria comprised of patients aged ≥ 14 years, those with poisoning via gastrointestinal
intake, and individuals with PQ plasma concentrations ≥ 0.01 mg/L by high-performance liquid chromatography. Exclusion criteria encompassed patients whose PQ concentrations were not detected
in the plasma, those with incomplete clinical data, those with other concomitant poisoning (such as alcohol poisoning), and pregnant women. For patients who developed symptoms within 6 h of
onset, gastric lavage was recommended. Those who developed symtoms within 12 h of onset were administered activated charcoal via oral or nasogastric route. Patients with positive
concentrations of PQ in blood tests were advised to undergo blood purification treatment, including blood perfusion, hemodialysis, or continuous venovenous hemofiltration. After excluding
contraindications such as gastrointestinal bleeding, all patients should be given methylprednisolone sodium succinate at a dosage of 80 mg/day for anti-inflammatory treatment through
intravenous infusion27. PREDICTORS We collected anonymous clinical data within 24 h of admission to the Emergency Department (ED) from electronic medical records. The collected data
comprised the following variables: time from poisoning to treatment at the study site, sex, age, level of consciousness (LOC), heart rate (HR), respiration rate (RR), systolic blood pressure
(SBP), diastolic blood pressure (DBP), white blood cell (WBC) count, neutrophil-to-lymphocyte ratio (NLR), monocyte-to-lymphocyte ratio (MLR), platelet count (PLT), total bilirubin, alanine
aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), blood urea nitrogen (BUN), creatinine, cystatin C, carbon dioxide combining power (CO2CP), blood
potassium, plasma PQ concentrations, and SIPP. Carbon dioxide combining power (CO2CP) measures a substance or solution's ability to react with and bind carbon dioxide (CO2) molecules.
It quantifies the substance's capacity to combine with CO2 chemically. The normal range of CO2CP typically falls between 22 and 30 milliequivalents per liter (mEq/L)28. Decreased LOC is
defined as a Glasgow Coma Scale (GCS) score below 1529. The severity of PQ poisoning was estimated quantitatively by using the SIPP, which was calculated by multiplying the elapsed time
(hours) from ingestion to arrival by the serum PQ level(μg/ml)11. All patient data were anonymized and de-identified. Variables were treated as continuous variables, except for sex and
disturbance of consciousness, which were binary variables. Finally, the continuous variables used to construct the nomogram were transformed into categorical variables based on the actual
data. This was achieved by categorizing them based on the normal upper and lower bounds, median, tertiles, and quartiles. CLINICAL OUTCOME The patients were divided into two groups: survivor
and non-survivor group, according to the occurrence of in-hospital death. The in-hospital mortality discussed in this study refers to all-cause mortality of APP patients, including deaths
in the ED. STATISTICAL ANALYSIS Data were analyzed using R version 4.2.1 (R Foundation for Statistical Computing, Vienna, Austria). A two-sided _P_ value < 0.05 indicated statistical
significance. Data are presented as medians (interquartile ranges) for continuous variables and as numbers (percentages) for categorical variables, as appropriate. The non-parametric
Mann–Whitney U test, chi-square analysis, and Fisher’s exact test were used to test for differences between groups, as appropriate. In this study, grouping was achieved through simple random
non-relaxation sampling using the simple_ra() function in the R software. All the baseline characteristics were comparable between the training and validation cohorts. In the training
cohort, the least absolute shrinkage and selection operator (LASSO) regression was used to decrease the potential collinearity of variables assessed from the same patient and the overfitting
of variables. The latent variables in the LASSO regression analysis were included in the multivariate analysis of stepwise forward selection to determine independent risk factors affecting
in-hospital mortality. The results are reported as odds ratios (ORs) and 95% confidence intervals (95% CI). A simple nomogram based on the independent risk factors was developed to predict
the probability of mortality. The prediction model was assessed using the concordance index (C-index), area under the receiver operating characteristic (ROC) curve (AUC), calibration curves,
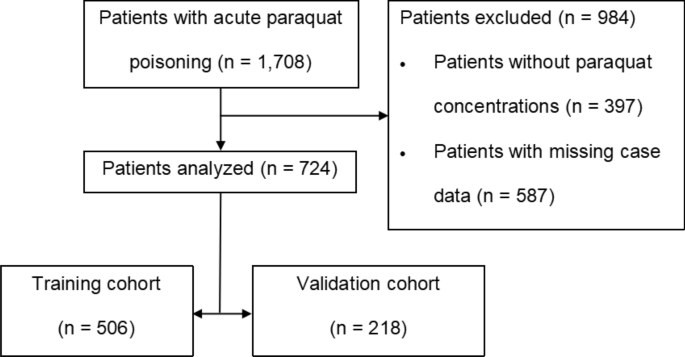
decision curve analysis (DCA), and clinical impact curves for both the training and validation cohorts30,31,32. RESULTS PATIENTS A total of 1708 patients with APP presented to our ED, of
whom 984 were excluded. Finally, 724 patients who met the inclusion criteria in this study were randomly divided into two groups: training and validation cohorts group using a 7:3 ratio
(Fig. 1); among them, 360/724 patients (49.7%) died. In terms of demographics, there were 325/724 (44.9%) males, with a median age of 33 (23–42) years. Among 506/724 patients in the training
group, 44.1% were male, with a median age of 29 (22–42) years, and 253/506 (50%) patients died. Of the 218/724 patients in the validation group, 46.8% were male, with a median age of 31
(23–42) years, and 107/218 (49.1%) patients died. No statistical significant differences were observed between the two groups (_P_ > 0.05) (Table 1). MODEL DEVELOPMENT In the demographic
and clinical characteristics analysis of 506 patients, 22 features were reduced to nine potential predictive features. These features had non-zero coefficients in the LASSO regression model,
which included age, LOC, WBC count, NLR, MLR, ALT, creatinine, CO2CP, and plasma PQ concentrations (Fig. 2a,b). CONSTRUCTION OF NOMOGRAM Multivariate analysis revealed several independent
risk factors for in-hospital mortality upon admission, including decreased LOC, NLR, ALT, creatinine, CO2CP, and plasma PQ concentrations. The detailed ORs and 95% CI of the multivariate
analysis are summarized in Table 2. Therefore, these six factors were used to construct the prediction model, as shown in Table 2. A simple nomogram was developed to predict the probability
of death based on the independent risk factors (Fig. 3a). The NLR values were categorized into quartiles as follows: category 1 for NLR < 6.2, category 2 for NLR 6.2–10.94, category 3 for
NLR 10.94–18.02, and category 4 for NLR > 18.02. ALT values were categorized based on the upper limit of normal as category 1 for ALT 0–39 IU/L and category 2 for ALT ≥ 40 IU/L.
Additionally, categorical variables were created based on the median value of creatinine, resulting in category 1 for creatinine ≤ 110 μmol/L and category 2 for creatinine > 110 μmol/L.
Finally, PQ values were categorized into quartiles: category 1 for PQ ≤ 0.33 g/mL, category 2 for PQ 0.33–1.66 g/mL, category 3 for PQ 1.66–11.15 g/mL, and category 4 for PQ > 11.15 g/mL.
As shown in the nomogram, each factor was assigned a point, and the total nomogram points were calculated by summing the individual points of all predictors. The relationship between the
total points and the probability of death is presented at the bottom of the nomogram. As an example, CO2CP was considered as a continuous variable. Where a decrease of 5 units corresponded
to an approximate 35 points increase in the risk score. The nomogram depicts the predicted probability of in-hospital death resulting from APP, measured on a scale of 0 to 300. Draw a
vertical line upward and assign labels to signify the respective points for each covariate. Repeated this procedure for each covariate to ascertain the cumulative. ASSESSMENT OF NOMOGRAM The
calibration curve in Fig. 3b,c suggests high consistency, demonstrating that the model’s predicted probabilities are close to the observed actual probabilities. The bias-corrected C-indices
for the training and validation cohorts were 0.933 and 0.947, respectively. In the training and validation cohorts, the C-indices were 0.953 (95% CI 0.936–0.970) and 0.947 (95% CI
0.920–0.974), respectively, indicating excellent accuracy. The ROC curves and AUC for the training and validation cohorts are shown in Fig. 4a,b. The DCA demonstrated that the nomogram had a
superior overall net benefit across a wide range of practical threshold probabilities (Fig. 5a,b). In addition, we plotted clinical impact curves to predict improved probability
stratification for a population size of 1000. The predicted probability coincided with the actual probability in the training and validation cohorts (Fig. 5c,d). CONSTRUCTION AND COMPARISON
OF OTHER PREDICTIVE MODELS Since plasma PQ concentrations accounted for much of the model and some primary hospitals could not detect them, we used LASSO and multivariate logistic regression
to construct another model without PQ plasma concentrations. Finally, we determined the following independent risk factors: LOC, age, WBC count, MLR, ALT Plasma PQ concentrations, and
CO2CP. Concurrently, we included the SIPP to compare the effectiveness of the three prediction models and construct the ROC curve (Fig. 6). DISCUSSION Timely identification of the severity
of patients with PQ poisoning is of utmost importance in clinical practice. It assists in formulating targeted treatment plans, allocating medical resources efficiently, and potentially
enhancing patient prognosis. Nevertheless, currently, no universally accepted method exists to comprehensively evaluate the correlation between clinical indicators and the survival outcomes
of patients with APP. This study bridges this gap by developing a prediction model that evaluates the risk of in-hospital mortality using clinical data collected during admission. The
created nomogram exhibited exceptional performance in both the training and validation cohorts, with an AUC exceeding 0.9. The included indicators can be readily acquired upon admission, and
the nomogram score can be calculated with ease. These characteristics render the model appropriate for swift and uncomplicated clinical implementation, facilitating early-stage evaluation
and prognosis prediction. Its convenience further encourages widespread adoption. Additionally, based on our comprehension, the sample size employed in this study is one of the largest
worldwide, and the constructed model has achieved near-perfect prediction performance. Moreover, after comparing the nomogram to SIPP, we have ascertained the superiority of our prediction
model. Prior nomogram models developed to predict the prognosis of APP patients primarily originated from the research conducted by Lu Shan. They retrospectively included a total of 80 cases
of patients with APP, utilizing serological markers and imaging examinations to construct their nomogram. The AUC in their training set and validation set were 0.953 (95% CI 0.936–0.970)
and 0.947 (95% CI 0.920–0.974), respectively. In comparison to their study, our current study encompasses an expanded sample size, a wider array of accessible indicators and demonstrates
exceptional predictive capacity. Our study revealed a high mortality rate in patients with decreased LOC due to APP, possibly related to toxic encephalopathy or hyperemia. Previous research
has reported that PQ can stimulate glutamate efflux, leading to excitotoxicity33. Animal studies have demonstrated PQ’s ability to induce α-synuclein upregulation, promote aggregate
formation, and activate microglial34. The NLR is a readily detectable inflammation marker using routine blood tests. It has been used to assess inflammatory-related lesions such as tumors,
ischemic stroke, and coronary artery disease35,36,37. Neutrophils are important members of the human immune system that have gradually gained attention for their role in APP. Studies have
shown that PQ can induce rapid expression of neutrophil chemoattractant proteins in bone marrow mesenchymal stem cells. Consequently, neutrophils rapidly increase in the blood and accumulate
in the lungs, promoting the production of chemokines and pro-inflammatory factors (such as tumor necrosis factor α and interleukin 8) and activating the inflammatory response.
Simultaneously, alveolar macrophages generated by neutrophils initiate immune responses and generate reactive oxygen species, leading to cellular nicotinamide adenine dinucleotide phosphate
(reduced coenzyme II) depletion and cell membrane lipid peroxidation, thereby promoting the expression of pro-fibrotic genes in fibroblasts and resulting in pulmonary fibrosis38,39.
Lymphocytes play a central role in regulating inflammatory responses in the human body. However, the specific mechanism through which PQ induces lymphocyte degradation remains unclear.
Whether PQ inhibits the cellular immune function requires further investigation. Our study showed that liver injury was an independent risk factor for death in patients with APP, which
contradicts the findings of previous studies showing that toxic hepatitis is common after PQ exposure. Toxic hepatitis appears mild and transient in scope but is associated with higher
complication rates, including respiratory and renal failure40. PQ penetration into tissues and organs can cause a series of oxidative stress reactions, generating a large amount of reactive
oxygen species and leading to organ damage or failure41. The kidney is the main excretory organ for PQ, eliminating 90% of PQ within 12–24 h. Consequently, patients with APP often present
with acute kidney injury characterized by elevated creatinine42. In addition, renal dysfunction can significantly reduce the PQ excretion rate and increase its accumulation in the body,
forming a vicious circle43,44. Further studies are needed to confirm whether correcting kidney injury improves prognosis. CO2CP refers to the plasma CO2 content measured after isolating
plasma from venous blood samples at room temperature and balancing it with the alveolar air of healthy people45. Two major acid–base disturbances, respiratory acidosis and metabolic
alkalosis, both of which can result in increased CO2CP, are common in patients with respiratory diseases. If respiratory diseases such as chronic obstructive pulmonary diseases are excluded,
the impact of the respiratory acid–base balance on CO2CP can be minimized46. In contrast, reduced CO2CP concentrations suggest metabolic acidosis47 or respiratory alkalosis48, both of which
are indicators of poor outcomes. Most often, decreased CO2CP indicates the presence of metabolic acidosis; however, it could also reflect a decline in bicarbonate concentration as
compensation for respiratory alkalosis. Therefore, it is considered a broad indicator of lung and kidney damage. In this study, the CO2CP of the mortality group was significantly lower than
that of the survival group (_P_ < 0.05). As the lungs and kidneys are important target organs of APP, this index can be regarded as a comprehensive evaluation index. Considering that some
primary hospitals cannot detect plasma PQ concentrations, we constructed a predictive model for plasma concentrations without PQ and compared it with SIPP. The second model showed the AUC
of 0.882 (95% CI 0.853–0.911). Although the performance of the second model is not as good as that of the first, it is still worth promoting. The current study has some limitations. First,
it was a single-center retrospective study with selection bias; furthermore, only 724 patients were included because of missing data, which may limit the scalability of the model. Second,
this study only included the clinical data of patients with APP at the time of enrollment and did not combine the clinical data after treatment to evaluate patient survival after discharge,
which may have affected the results. Owing to limitations in clinical data, the GCS classification in this study was restricted to < 15/15. However, in future studies, we aim to collect
more comprehensive data to improve nomogram precision. Moreover, because of the severe lack of clinical data and absence of indicators of lung injury, this study was performed
retrospectively. The model must be updated when more multicenter data become available. CONCLUSION We developed a predictive model for in-hospital mortality in patients with APP. The
nomogram, which includes six risk factors with favorable predictive accuracy, discrimination, and clinical utility, allows for simple and rapid individual patient risk estimates. It can be
used on admission to the ED to predict mortality and facilitate early risk stratification and actionable measures in clinical practice after further external validation. DATA AVAILABILITY
The datasets generated during and/or analyzed during the current study are not publicly available due to data confidentiality. However, they can be obtained from the corresponding author
upon reasonable request. REFERENCES * Wilks, M. F. _et al._ Improvement in survival after paraquat ingestion following introduction of a new formulation in Sri Lanka. _PLoS Med._ 5, e49
(2008). Article PubMed PubMed Central Google Scholar * Gawarammana, I. B. & Buckley, N. A. Medical management of paraquat ingestion. _Br. J. Clin. Pharmacol._ 72, 745–757 (2011).
Article CAS PubMed PubMed Central Google Scholar * Wu, W.-P. _et al._ Addition of immunosuppressive treatment to hemoperfusion is associated with improved survival after paraquat
poisoning: A nationwide study. _PLoS One_ 9, e87568 (2014). Article ADS PubMed PubMed Central Google Scholar * Gao, J. _et al._ Prolonged methylprednisolone therapy after the pulse
treatment for patients with moderate-to-severe paraquat poisoning: A retrospective analysis. _Medicine (Baltimore)_ 96, e7244 (2017). Article CAS PubMed Google Scholar * Tan, J. T. _et
al._ Paraquat poisoning: Experience in hospital taiping (year 2008–October 2011). _Med. J. Malaysia_ 68, 384–388 (2013). CAS PubMed Google Scholar * Wang, W. J. _et al._ Sequential organ
failure assessment in predicting mortality after paraquat poisoning: A meta-analysis. _PLoS One_ 13, e0207725 (2018). Article PubMed PubMed Central Google Scholar * Gheshlaghi, F. _et
al._ Prediction of mortality and morbidity following paraquat poisoning based on trend of liver and kidney injury. _BMC Pharmacol. Toxicol._ 23, 67 (2022). Article CAS PubMed PubMed
Central Google Scholar * Li, C. _et al._ Treatment outcome of combined continuous venovenous hemofiltration and hemoperfusion in acute paraquat poisoning: A prospective controlled trial.
_Crit. Care Med._ 46, 100–107 (2018). Article ADS CAS PubMed Google Scholar * Wang, Y. _et al._ Clinical effect of haemoperfusion combined with continuous veno-veno haemofiltration in
treatment of paraquat poisoning: A meta-analysis. _Zhonghua Wei Zhong Bing Ji Jiu Yi Xue_ 31, 214–220 (2019). PubMed Google Scholar * Hsu, C.-W. _et al._ Early hemoperfusion may improve
survival of severely paraquat-poisoned patients. _PLoS One_ 7, e48397 (2012). Article ADS CAS PubMed PubMed Central Google Scholar * Sawada, Y. _et al._ Severity index of paraquat
poisoning. _Lancet (London, England)_ 1(8598), 1333 (1988). Article CAS PubMed Google Scholar * Proudfoot, A. T. _et al._ Paraquat poisoning: Significance of plasma-paraquat
concentrations. _Lancet (London, England)_ 2(8138), 330–332 (1979). Article CAS PubMed Google Scholar * Senarathna, L. _et al._ Prediction of outcome after paraquat poisoning by
measurement of the plasma paraquat concentration. _QJM Mon. J. Assoc. Phys._ 102(4), 251–259 (2009). CAS Google Scholar * Xu, S. _et al._ APACHE score, severity index of paraquat
poisoning, and serum lactic acid concentration in the prognosis of paraquat poisoning of Chinese patients. _Pediatr Emerg Care_ 31(2), 117–121 (2015). Article PubMed Google Scholar *
Suzuki, K. _et al._ Evaluation of severity indexes of patients with paraquat poisoning. _Hum. Exp. Toxicol._ 10(1), 21–23 (1991). Article CAS PubMed Google Scholar * Chen, H. _et al._ An
effective machine learning approach for prognosis of paraquat poisoning patients using blood routine indexes. _Basic Clin. Pharmacol. Toxicol._ 120(1), 86–96 (2017). Article MathSciNet
CAS PubMed Google Scholar * Gheshlaghi, F. _et al._ Prediction of mortality and morbidity following paraquat poisoning based on trend of liver and kidney injury. _BMC Pharmacol. Toxicol._
23(1), 67 (2022). Article CAS PubMed PubMed Central Google Scholar * Hu, L. _et al._ A new machine-learning method to prognosticate paraquat poisoned patients by combining coagulation,
liver, and kidney indices. _PloS One_ 12(10), e0186427 (2017). Article PubMed PubMed Central Google Scholar * Zhao, Y. _et al._ Serum anion gap at admission as a predictor of the
survival of patients with paraquat poisoning: A retrospective analysis. _Medicine_ 99(31), e21351 (2020). Article CAS PubMed PubMed Central Google Scholar * Lu, S. _et al._ Development
and validation of a radiomics nomogram for prognosis prediction of patients with acute paraquat poisoning: A retrospective cohort study. _BioMed Res. Int._ 2021, 6621894 (2021). ADS PubMed
PubMed Central Google Scholar * Huang, J. _et al._ The value of APACHE II in predicting mortality after paraquat poisoning in Chinese and Korean population: A systematic review and
meta-analysis. _Medicine_ 96(30), e6838 (2017). Article CAS PubMed PubMed Central Google Scholar * Wang, W. J. _et al._ Sequential organ failure assessment in predicting mortality after
paraquat poisoning: A meta-analysis. _PloS One_ 13(11), e0207725 (2018). Article PubMed PubMed Central Google Scholar * Persson, H. E. _et al._ Poisoning severity score grading of acute
poisoning. _J. Toxicol. Clin. Toxicol._ 36(3), 205–213 (1998). Article CAS PubMed Google Scholar * Wang, J. _et al._ Identify the early predictor of mortality in patients with acute
paraquat poisoning. _BioMed Res. Int._ 2020, 8894180 (2020). Article PubMed PubMed Central Google Scholar * Iasonos, A. _et al._ How to build and interpret a nomogram for cancer
prognosis. _J. Clin. Oncol._ 26, 1364–1370 (2008). Article PubMed Google Scholar * Lu, S. _et al._ Development and validation of a radiomics nomogram for prognosis prediction of patients
with acute paraquat poisoning: A retrospective cohort study. _Biomed. Res. Int._ 2021, 6621894 (2021). ADS PubMed PubMed Central Google Scholar * Li, C. _et al._ Treatment outcome of
combined continuous venovenous hemofiltration and hemoperfusion in acute paraquat poisoning: A prospective controlled trial. _Crit. Care Med._ 46(1), 100–107 (2018). Article ADS CAS
PubMed Google Scholar * Huang, P.-F. _et al._ Predictive value of admission CO2 combining power combined with serum sodium for the prognosis in acute Stanford type A aortic dissection
patients. _Sci. Rep._ 13(1), 1048 (2023). Article ADS CAS PubMed PubMed Central Google Scholar * Savonius, O. _et al._ PCR-confirmed malaria among children presenting with a decreased
level of consciousness in Angola: A prospective, observational study. _Malar. J._ 22(1), 130 (2023). Article PubMed PubMed Central Google Scholar * Caetano, S. J., Sonpavde, G. &
Pond, G. R. C-statistic: A brief explanation of its construction, interpretation and limitations. _Eur. J. Cancer_ 90, 130–132 (2018). Article CAS PubMed Google Scholar * Alba, A. C. _et
al._ Discrimination and calibration of clinical prediction models: Users’ guides to the medical literature. _JAMA_ 318, 1377–1384 (2017). Article PubMed Google Scholar * Vickers, A. J.
& Elkin, E. B. Decision curve analysis: A novel method for evaluating prediction models. _Med. Decis. Mak._ 26, 565–574 (2006). Article Google Scholar * Shi, C. _et al._ Bone marrow
mesenchymal stem and progenitor cells induce monocyte emigration in response to circulating toll-like receptor ligands. _Immunity_ 34, 590–601 (2011). Article CAS PubMed PubMed Central
Google Scholar * Yang, C.-J. _et al._ Spectrum of toxic hepatitis following intentional paraquat ingestion: Analysis of 187 cases. _Liver Int._ 32, 1400–1406 (2012). Article CAS PubMed
Google Scholar * Nouri, A., Heibati, F. & Heidarian, E. Gallic acid exerts anti-inflammatory, anti-oxidative stress, and nephroprotective effects against paraquat-induced renal injury
in male rats. _Naunyn Schmiedebergs Arch. Pharmacol._ 394, 1–9 (2021). Article CAS PubMed Google Scholar * Sun, B. & He, Y. Paraquat poisoning mechanism and its clinical treatment
progress. _Zhonghua Wei Zhong Bing Ji Jiu Yi Xue_ 29, 1043–1046 (2017). PubMed Google Scholar * Mohamed, F. _et al._ Mechanisms underlying early rapid increases in creatinine in paraquat
poisoning. _PLoS One_ 10, e0122357 (2015). Article PubMed PubMed Central Google Scholar * Kim, S.-J. _et al._ The clinical features of acute kidney injury in patients with acute paraquat
intoxication. _Nephrol. Dial. Transpl._ 24, 1226–1232 (2009). Article CAS Google Scholar * Proudfoot, A. T. _et al._ Paraquat poisoning: Significance of plasma-paraquat concentrations.
_Lancet_ 2, 330–332 (1979). Article CAS PubMed Google Scholar * Hart, T. B., Nevitt, A. & Whitehead, A. A new statistical approach to the prognostic significance of plasma paraquat
concentrations. _Lancet_ 2, 1222–1223 (1984). Article CAS PubMed Google Scholar * Scherrmann, J. M. _et al._ Prognostic value of plasma and urine paraquat concentration. _Hum. Toxicol._
6, 91–93 (1987). Article CAS PubMed Google Scholar * Jones, A. L., Elton, R. & Flanagan, R. Multiple logistic regression analysis of plasma paraquat concentrations as a predictor of
outcome in 375 cases of paraquat poisoning. _QJM_ 92, 573–578 (1999). Article CAS PubMed Google Scholar * Yamamoto, I. _et al._ Correlating the severity of paraquat poisoning with
specific hemodynamic and oxygen metabolism variables. _Crit. Care Med._ 28, 1877–1883 (2000). Article CAS PubMed Google Scholar * Li, Y., Zhang, H. & Zhang, G. Early white blood cell
count in predicting mortality after acute paraquat poisoning: A meta-analysis. _Zhonghua Wei Zhong Bing Ji Jiu Yi Xue_ 31, 1013–1017 (2019). PubMed Google Scholar * Li, S. _et al._ The
significance of CO2 combining power in predicting prognosis of patients with stage II and III colorectal cancer. _Biomark. Med._ 13, 1071–1080 (2019). Article CAS PubMed Google Scholar *
Hu, J. _et al._ Metabolic acidosis as a risk factor for the development of acute kidney injury and hospital mortality. _Exp. Ther. Med._ 13, 2362–2374 (2017). Article CAS PubMed PubMed
Central Google Scholar * Gunnerson, K. J. _et al._ Lactate versus non-lactate metabolic acidosis: A retrospective outcome evaluation of critically ill patients. _Crit. Care_ 10, R22
(2006). Article PubMed PubMed Central Google Scholar * Shirakabe, A. _et al._ Clinical significance of acid-base balance in an emergency setting in patients with acute heart failure. _J.
Cardiol._ 60, 288–294 (2012). Article PubMed Google Scholar Download references ACKNOWLEDGEMENTS This work was supported by the National Natural Science Foundation of China (Grant No.
82072156) and the Science and Technology Department of Sichuan Province (Grant No. 2022YFS0273). AUTHOR INFORMATION Author notes * These authors contributed equally: Guo Tang and Zhen Jiang.
AUTHORS AND AFFILIATIONS * Emergency Medicine Laboratory and the Department of Emergency, West China Hospital, Sichuan University, No. 37 Guoxue Alley, Chengdu, 610041, Sichuan, China Guo
Tang, Zhen Jiang, Lingjie Xu, Ying Yang, Sha Yang & Rong Yao Authors * Guo Tang View author publications You can also search for this author inPubMed Google Scholar * Zhen Jiang View
author publications You can also search for this author inPubMed Google Scholar * Lingjie Xu View author publications You can also search for this author inPubMed Google Scholar * Ying Yang
View author publications You can also search for this author inPubMed Google Scholar * Sha Yang View author publications You can also search for this author inPubMed Google Scholar * Rong
Yao View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS G.T. performed all data analysis. G.T. and J.Z. wrote the main manuscript text. J.X.,
Y.Y. and S.Y. prepared all figures and table. RY conducted the design and review of the article. All authors reviewed the manuscript. CORRESPONDING AUTHOR Correspondence to Rong Yao. ETHICS
DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard to jurisdictional claims
in published maps and institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits
use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the
Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless
indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory
regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Tang, G., Jiang, Z., Xu, L. _et al._ Development and validation of a prognostic
nomogram for predicting in-hospital mortality of patients with acute paraquat poisoning. _Sci Rep_ 14, 1622 (2024). https://doi.org/10.1038/s41598-023-50722-z Download citation * Received:
16 July 2023 * Accepted: 23 December 2023 * Published: 18 January 2024 * DOI: https://doi.org/10.1038/s41598-023-50722-z SHARE THIS ARTICLE Anyone you share the following link with will be
able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative