Exploring the contribution of lifestyle to the impact of education on the risk of cancer through mendelian randomization analysis
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Educational attainment (EA) has been linked to the risk of several types of cancer, despite having no expected direct biological connection. In this paper, we investigate the
mediating role of alcohol consumption, smoking, vegetable consumption, fruit consumption and body mass index (BMI) in explaining the effect of EA on 7 cancer groupings. Large-scale genome
wide association study (GWAS) results were used to construct the genetic instrument for EA and the lifestyle factors. We conducted GWAS in the UK Biobank sample in up to 335,024 individuals
to obtain genetic association data for the cancer outcomes. Univariable and multivariable two-sample Mendelian randomization (MR) analyses and mediation analyses were then conducted to
explore the causal effect and mediating proportions of these relations. MR mediation analysis revealed that reduced lifetime smoking index accounted for 81.7% (49.1% to 100%) of the
protective effect of higher EA on lower respiratory cancer. Moreover, the effect of higher EA on lower respiratory cancer was mediated through vegetable consumption by 10.2% (4.4% to 15.9%).
We found genetic evidence that the effect of EA on groups of cancer is due to behavioural changes in avoiding well established risk factors such as smoking and vegetable consuming. SIMILAR
CONTENT BEING VIEWED BY OTHERS THE MEDIATING ROLE OF COMBINED LIFESTYLE FACTORS ON THE RELATIONSHIP BETWEEN EDUCATION AND GASTRIC CANCER IN THE STOMACH CANCER POOLING (STOP) PROJECT Article
27 May 2022 PHEWAS-BASED CLUSTERING OF MENDELIAN RANDOMISATION INSTRUMENTS REVEALS DISTINCT MECHANISM-SPECIFIC CAUSAL EFFECTS BETWEEN OBESITY AND EDUCATIONAL ATTAINMENT Article Open access
15 February 2024 MENDELIAN RANDOMIZATION EVIDENCE FOR THE CAUSAL EFFECTS OF SOCIO-ECONOMIC INEQUALITY ON HUMAN LONGEVITY AMONG EUROPEANS Article 29 June 2023 INTRODUCTION Cancer is a risk to
health and the primary cause of death worldwide1,2. An estimated 19.3 million new cancer cases and almost 10.0 million cancer deaths occurred in 20203. A steady increase in mortality and
incidence, particularly in developed countries4, calls for more effective cancer prevention5. While improvements in survival rates reflect progress in medical technology and healthcare, the
rising incidence of cancer has been attributed to generational changes in obesity, lowered physical activity, a difference in diet and other lifestyle factors6,7. The fact that increasing
cancer rates offset higher survival rates and lead to higher absolute mortality8 signifies the importance of tackling modifiable risk factors that lead to high incidence rates. Educational
attainment (EA) predicts cancer outcomes9,10,11 and is a central driver between socio-economic status (SES) and health12. For example, 22% of US cancer deaths could be prevented if all
Americans had the cancer death rates of college-educated Americans5. Although there is no direct biological link between EA and cancer risk, it is believed that EA leads to more effective
self-management and habits12. Various behaviourally related modifiable risk factors in low EA/SES have been studied. The most prominent factors identified include: alcohol consumption,
physical inactivity, obesity, cigarette smoking, as well as low fruit and vegetable consumption13,14,15,16,17. Studies specifically targeting the mediating effect of modifiable risk factors
in EA and cancer risk are sparse. Some studies assess all-cause mortality18,19 while other studies address general cancer risk in the context of SES20,21,22. Quantitative assessments of the
mediating effect of risk factors from such observational data are based on multivariable analysis, a method aiming to disentangle the effects of multiple variables on the outcome23. While
the results of such assessments provide direction for further experimental studies, their validity is limited. Observational studies are also at risk of systemic biases between groups24.
Furthermore, clustering of risky behaviours is common25, whereby individuals readily engage with a variety of behaviours, both risky and mitigating, the negative outcome that the authors
wish to investigate, potentially introducing further bias. This makes it difficult to measure and correct for all risky behaviours and unmeasured risk factors might constitute confounders26
using traditional experimental investigative techniques. Moreover, data is commonly collected at distinct times, which does not capture a lifetime exposure27. Mendelian Randomization is an
analytical method to assess whether a risk factor has a causal effect on an outcome of interest, using genetic variants as instrumental variables28. The approach treats genetic variants as
proxy measures for clinical interventions on risk factors, and thus has been extensively shown to anticipate the results of a randomised control trial29. The aim of this study was to explore
the mediating role of lifestyle factors in explaining the effect of EA on the risk of cancer, using individual level data from the UK Biobank (UKB) and summary statistics from reliable
published genome wide association studies (GWAS). We considered five lifestyle factors individually and simultaneously: number of alcoholic drinks per week, lifetime smoking index, BMI,
fruit consumption and vegetable consumption. These five lifestyle factors were used to interrogate the underlying mechanisms by which EA affects the risk of cancer. METHODS DATA SOURCES
OUTCOME DATA SOURCE Individual level data on cancer incidence were obtained from UK Biobank (UKB), a prospective population study with detailed information about approximately 500,000
participants30. The data was collected between 2006 and 2011 and participants volunteered to provide biological samples for the measurement of biochemical markers and subsequent genotyping,
anthropomorphic measures through a number of collection centres in the UK, and sociodemographic, lifestyle and health behaviours information through a series of in-person and online
questionnaires. The processes for genotyping and data management have been described in depth31. Phenotype data were obtained from UKB data-fields 41,270 and 40,006 for 30 site-specific
cancers. In this analysis, up to 335,024 UKB participants of European ancestry were considered, after excluding samples with relatedness of first or second degree and samples with discordant
genetic and reported sex. Cancer cases were defined according to ICD-10 codes (international classification of diseases, 10th revision), obtained through linkage to national cancer
registries. Genetic associations were estimated with 7 cancer groupings: digestive (7695 cases and 327,356 controls), female reproductive (3,612 cases and 177,190 controls), head and neck
(1331 cases and 334,378 controls), lower gastrointestinal (GI) tract (6545 cases and 328,601 controls), lower respiratory (2307 cases and 332,457 controls), male reproductive (8988 cases and
149,131 controls) and upper gastrointestinal (GI) tract (1300 cases and 149,131 controls). Cancer groupings were generated to maximise statistical power and were determined based on their
location in the body. Those with a cancer diagnosis were considered a case only for their chronologically first reported cancer type. This was done to distinguish between primary cancers
originating in the specific tissue and secondary cancers metastasising from another location. EXPOSURE DATA SOURCE To assess EA, we used publicly available summary statistics from a Social
Science Genetic Association Consortium (SSGAC) meta-analysis of GWAS32. The primary meta-analysis combined 3 quality-controlled cohort-level results from studies in Europe and USA and was
conducted on approximately 3 million individuals of European ancestry. Education years were measured for all samples over the age of 30. In this work, we used meta-analysis GWAS results from
all discovery cohorts except 23andMe, conducted on approximately 800,000 samples, 442,183 of which were UKB participants. LIFESTYLE DATA SOURCES Alcohol consumption, body mass index (BMI),
smoking, fruit and vegetable consumption were assessed as potentially mediating risk factors in this work. Publicly available summary level data were used for alcoholic drinks consumed per
week from Saunders et al.33,34, who conducted a GWAS meta-analysis using data from 60 cohorts on 2,965,643 individuals. To capture smoking behaviour, we used genetic summary statistics on
lifetime smoking index, measured in 462,690 UKB participants of European ancestry who had phenotype data and passed genotype inclusion criteria35. Following a method previously reported36,
smoking status, age at initiation in years, age at cessation in years and number of cigarettes smoked per day were combined into a lifetime smoking index. Genetic estimates for BMI were
obtained from the Genetic Investigation of Anthropometric Traits consortium (GIANT) GWAS meta-analysis of 681,275 samples of European ancestry37, around 450,000 of which were UKB
participants. To assess fruit and vegetable consumption, we used publicly available GWAS summary statistics from the MR-base38 for binary traits ‘fruit consumers’ and ‘vegetable consumers’,
conducted on 64,949 UKB participants of European ancestry. These variables were generated as consumption over the last 24 h. A number of _Yes/No_ questions relating to eating particular food
groups or items were given to the participants following the pattern ‘_Did you eat any_ < _food-group_ > _yesterday?_’ providing examples of relevant foods and a picture. The
participants completed the questionnaire in the assessment centre or online in four separate occasions within a year (http://biobank.ctsu.ox.ac.uk/crystal/docs/DietWebQ.pdf). GENOME WIDE
ASSOCIATION STUDIES Genome wide association studies were conducted to obtain associations between 9,420,314 genotyped and imputed single nucleotide polymorphisms (SNPs) and 7 cancer
groupings: digestive, female reproductive, head and neck, lower GI, lower respiratory, male reproductive and upper GI in up to 335,024 unrelated participants of white British ancestry. The
SNPs tested were located in the autosomes. Only SNPs with a minor allele frequency greater than 0.01 and a Hardy–Weinberg equilibrium p-value greater than 10–6 were considered. The
association of each SNP was tested using a linear regression model, adjusting for sex, age and the first 4 genetic principal components to control for population structure. STATISTICAL
ANALYSIS GENETIC INSTRUMENTS Genetic variants were considered as instrumental variables for EA and the lifestyle factors in the MR analysis if they were bi-allelic, had a minor allele
frequency (MAF) greater than 0.01 and a Hardy–Weinberg equilibrium P-value greater than 10–6. For EA, lifetime smoking index, drinks per week and BMI, genetic variants below the genome-wide
significance threshold (p < 5 × 10–8) were selected as instruments, whereas for fruit and vegetable consumption, we considered a less stringent p-value threshold (p < 10–5), as it was
the lowest threshold that provided robust signals for the two dietary GWAS results. We identified independent SNPs after clumping summary estimates, using a linkage disequilibrium (LD)
threshold of r2 < 0.001 and a clumping window of 10 Mb. For the LD estimates between the genetic variants, we used the 1000 genomes phase 3 European reference panel39. To ensure that the
first MR assumption holds, only genetic variants with an F-statistic greater than 10 were included in the analysis40. To further test the validity of the MR assumptions, we identified traits
that associate with the genetic instruments used in this work, using the SNP nexus platform (https://www.snp-nexus.org/v4/). In the two-sample MR setting, genetic variants were excluded
from the analysis when the direction of effects between exposure and outcome associations could not be inferred (in the case of palindromic SNPs with MAF greater than 0.42). Genetic
instruments comprised 413 independent SNPs for EA, 507 independent variants for BMI, 126 for lifetime smoking index, 10 for drinks per week, 32 for fruit consuming and 21 for vegetable
consuming. TWO-SAMPLE UNIVARIABLE MENDELIAN RANDOMIZATION The current study used two-sample univariable and multivariable MR analysis in a multistep process to assess the relationships
between EA, each of the 5 lifestyle factors and 7 cancer outcomes of interest. First, we tested the associations between EA and 7 cancer categories. We used categories over site-specific
cancer types to maximise statistical power. Following this, we assessed the associations of EA with the 5 possible mediating lifestyle factors. Lifestyle factors were then tested against the
7 cancer categories simultaneously through MVMR. The random-effects inverse variance weighted (IVW) method was used as the main method for univariable MR analysis, which provides precise
causal estimates, under the assumption that all genetic variants are valid instrumental variables41. The three instrumental variable assumptions dictate that the genetic instrument is
associated with the exposure, is independent of any confounders of the exposure and outcome association and is associated with the outcome only via the exposure. In sensitivity analysis, we
conducted the MR-Egger method42 to detect possible violations due to pleiotropic effects within genetic variants in the analysis and MR-weighted median method, which reports an accurate
effect estimate, given that at least 50% of the weight in the analysis comes from valid instruments43. In addition, we performed MR-PRESSO, a method which detects and removes outlier SNPs
based on their contribution to heterogeneity44. The I2 statistic was calculated to detect heterogeneity among the MR estimates obtained from multiple genetic variants. MR-IVW effect
estimates were deemed statistically significant if association p-values were smaller than the Bonferroni corrected threshold 0.05/n, where n represents the total number of independent tests
in each part of the analysis (EA with 7 cancer groups: _p_ < 0.05/7 = 7.14 × 10–3, EA with 5 lifestyle factors: _p_ < 0.01 and 5 lifestyle factors and EA with 7 cancer groups: _p_ <
1.19 × 10–3). To quantify the amount of bias due to sample overlap in the MR effect estimates, we used the MRlap method45, which estimates corrected MR estimates, accounting for potential
bias. We reported the p-value corresponding to the test statistic used to test for differences between the observed and corrected MR estimates. TWO-SAMPLE MULTIVARIABLE MENDELIAN
RANDOMIZATION To estimate the direct effect of each of the lifestyle factors on the risk of cancer, we performed multivariable Mendelian randomization (MVMR) analysis46. This method allows
the use of multiple genetic variants associated with more than one risk factor as instruments to identify the causal effect of each risk factor on the outcome, independent of the rest of the
risk factors. To obtain the list of genetic instruments for MVMR, we first merged, before clumping, the SNPs associated with each lifestyle factor or the exposure below their respective
p-value thresholds as determined in the univariable MR analysis, and then clumped these genetic variants using for each variant the smallest p-value of their association with each lifestyle
factor. Following this process, we generated a list of independent genetic variants that are associated with at least one lifestyle factor, as the MVMR paradigm dictates. All estimates were
reported as odds ratio (OR) per unit increase in exposure, together with their 95% confidence interval (95% CI). PROPORTION OF LIFESTYLE FACTOR MEDIATION Network Mendelian randomization
(network-MR) was conducted47 using the MR effect estimates and standard errors obtained previously to calculate the direct and indirect effect of EA on cancer risk. This was done per cancer
outcome and per possible mediator, using their MVMR estimates, to obtain fractions of mediated and non-mediated effects. The indirect effect of EA on cancer through a lifestyle factor was
estimated by multiplying the effect of EA on that factor times the effect of the lifestyle factor on the cancer outcome. The total effect was the estimated MR effect of EA on cancer. The
direct effect was estimated by subtracting the indirect effect from the total effect, estimated from the first step MR. Last, the mediation proportion for each lifestyle factor was
calculated by dividing the indirect effect over the total effect. To derive standard errors of the mediation proportion estimates, we used the delta method48. STATISTICAL SOFTWARE Analysis
was conducted in R version 4.0.249, two-sample analyses and sensitivity analyses were performed using the “TwoSampleMR” v.0.5.650 and “MRPRESSO” v1.044 R packages. Figures were produced
using the R package “forestplot” v3.1.151. GWAS was conducted using PLINK 1.90 command line tool (www.cog-genomics.org/plink/1.9/). This study is reported based on the Strengthening the
Reporting of Observational Studies in Epidemiology (STROBE) guidelines Supplementary Table 1. ETHICS APPROVAL This study is based on publicly available data and the informed consent and
ethical review were acquired in all the original studies. The study is reported following the STROBE-MR statement. RESULTS Individual SNP estimates of the per-allele effects on EA, BMI,
lifetime smoking index, drinks per week, fruit consumption and vegetable consumption are reported in Supplementary Table 2. Sample size, number of cases and included ICD-10 codes per cancer
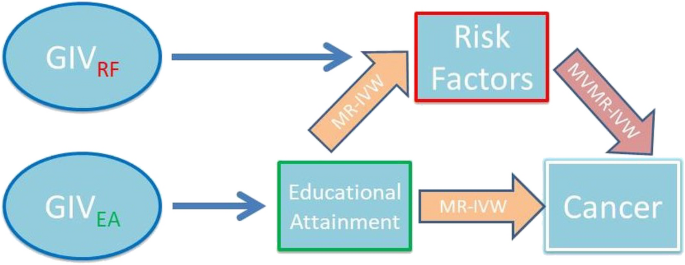
group are shown in Supplementary Table 3. Graphical representation of the model can be found in the directed acyclic graph (DAG) in Fig. 1. Univariable and multivariable MR estimates,
sensitivity analysis results, MR-Egger intercepts and genetic heterogeneity statistics are provided in Supplementary Tables 4–6. Associations were considered statistically significant in
this work if MR-IVW and MR-PRESSO estimates were significant after multiple testing correction, MR-Egger and MR-weighted median estimates had the same direction of effect and MR-Egger
intercept was not significant (p > 0.05). The associations of EA with the lifestyle factors and the various groups of cancer were obtained per one standard deviation (sd) increase, which
corresponds to 3.6 years of additional education in the UKB. MR-IVW results revealed two associations between EA and the odds of cancer, which were below the Bonferroni adjusted p-value
threshold of p = 0.05/7 = 7.14 × 10–3. One sd increase in EA was associated with lower odds of lower respiratory tract cancer (OR: 0.40, 95% CI: 0.30 to 0.54), lower odds of upper GI cancer
(OR: 0.59, 0.43 to 0.82) and lower odds of digestive cancer (OR: 0.81, 0.69 to 0.94) (Fig. 2). At a p = 0.05/5 = 0.01 Bonferroni threshold, MR-IVW results indicated that increasing EA was
associated with lower BMI (beta: -0.24, -0.28 to -0.20) and lower lifetime smoking index (beta: -0.22, -0.24 to -0.20). One sd increase in EA was also associated with increased odds of fruit
(OR: 1.12, 1.10 to 1.14) and vegetable consumption (OR: 1.08, 1.07 to 1.11). Weighted median and MR-Egger effects had similar effect estimates to the IVW method. The effect of EA on BMI was
quite heterogeneous with an I2 statistic of 89% but a consistent effect between the MR-IVW, MR-Weighted median and MR-PRESSO methods. MR results of the effect of higher genetically
predicted EA on lifestyle factors are summarised in Fig. 3. Moreover, using SNP nexus, the identified traits are unlikely to be potential confounders of the associations tested
(Supplementary Table 7). MRlap method results suggested that there was no significant effect of sample overlap in the calculated MR estimates (Supplementary Table 8). MVMR analysis revealed
seven significant effects of the lifestyle factors on the risk of cancer groups, below p = 0.05/42 = 1.19 × 10–3. Increasing lifetime smoking index was associated with increasing odds of
lower respiratory cancer (OR: 31.4, 17.8 to 55.6), head and neck cancer (OR: 5.96, 2.95 to 12.03), upper gastrointestinal cancer (OR: 4.12, 2.20 to 7.70) and digestive cancer (OR: 1.67, 1.25
to 2.24). Higher genetically predicted BMI was associated with increased odds of upper GI cancer (OR: 1.64, 1.30 to 2.07) and lower respiratory cancer (OR:1.65, 1.31 to 2.07). Last,
vegetable consuming was associated with lower odds of lower respiratory cancer (OR: 0.32, 0.21 to 0.52) (Fig. 4). MR mediation analysis revealed two significant mediation ratios, after
correcting for multiple testing (Supplementary Table 9). The largest part of the protective effect of increased EA on lower odds of lower respiratory cancer was mediated through smoking by
81.7% (50.5 to 100%). Interestingly, vegetable consumption was also mediating factor for the link between EA and lower respiratory cancer with 10.2% (4.4 to 15.9%). DISCUSSION The objective
of this work was to identify the mediating lifestyle factors linking educational attainment to risk of cancer. We investigated 5 well supported lifestyle factors, including alcohol
consumption, body mass index, fruit consumption, lifestyle smoking and vegetable consumption, using summary statistics from large cohorts. In our genetic analysis we found evidence of
associations between EA and lower respiratory, upper GI and digestive cancers. MR mediation analysis results revealed that on average, 81.7% of the protective effect of EA on lower
respiratory cancer was mediated by lifetime smoking and 10.2% by vegetable consumption. The findings for the effect of EA on cancer risk are largely in agreement with literature. All
potential causal associations found agree with estimates based on observational data found in literature5,52,53,54. MR methods therefore provide high-level evidence for a causal relationship
between EA and cancer. Regarding the effect of EA on the lifestyle factors, the current study also supports existing findings. Previous comparisons of high-school and college EA estimated a
56%55 and 64%56 lower smoking status. Observational studies indicate that there is a negative association between EA and BMI in higher-income countries57. Existing literature provides no
association estimates of EA with fruit and vegetable consumption but suggests an effect of socio-economic status on fruit and slightly lower on vegetable consumption58,59. Moreover,
increasing EA is associated with increased alcohol intake frequency in MR studies60. The MR estimates of smoking on lower respiratory cancer are in agreement with observational
studies61,62,63 for current smokers, but also with existing MR studies64. Moreover, genetically predicted BMI has been found to be positively associated with the risk of oesophageal
cancer65,66. Previous observational study on mediation analysis for EA on lung cancer found that, adjustment for smoking decreased relative educational differences of lung cancer incidence
by 50 to 70%67. Our MR network study gives a comparable mediation proportion of the protective effect of EA on lower respiratory cancer through lifetime exposure to smoking, around 80% of
the total effect, since MR corrects for unknown confounding factors. Last, we identified a significant mediation proportion of the protective effect of EA on lung cancer through vegetable
consumption, which is consistent with numerous observational studies suggesting a protective role of fruit and vegetables in lung cancer aetiology68,69,70. Prior to making a number of key
conclusions based on the data presented in this study, it is prudent to consider some of the limitations. The UKB sample has been shown to not be fully representative of the UK population.
Individuals in the UKB have a higher likelihood, compared to the UK population, of being lean, non-smokers, non-drinkers and being older and female. This “healthy volunteer” bias is also
affecting the total cancer incidence which may have introduced bias in our results. However, the assessment of effects of exposures on health outcomes in non-representative samples is still
generalisable71. Fruit and vegetable consumption GWAS did not yield many strong genetic instruments compared to other factors possibly due to self-reported information and the limited time
this accounts for. In addition, we excluded UKB participants from the lifestyle factors, where possible. However, partial sample overlap of EA, BMI, fruit and vegetable consumption with
cancer incidence may have biased some of the MR effect estimates away from the null. Moreover, MR makes the assumption that all associations are linear, however, existing studies have shown
a J-shape association between alcohol consumption and cancer risks72,73. In addition, the diagnosis of one cancer type may affect the surveillance, screening, or diagnostic practices for
other cancer types. This could introduce bias if the ascertainment of the second cancer is influenced by the awareness or diagnosis of the first cancer. Last, due to the lack of availability
of individual level data, we couldn’t test if there were any interactions between the exposure and the mediators. The statistical power of a MR study depends on how much variation in the
exposure is explained by the chosen genetic instrumental variables, the sample size and the true causal association between exposure and outcome74. Low cancer prevalence limits statistical
power in this study, which may in turn explain the lack of statistically significant mediators identified beyond smoking and BMI. In addition, low vegetable and fruit consumption
heritability could be limiting the usefulness of their genetic instruments. MVMR corrects for overlap in pathways, however, it is not able to deal with unmeasured confounders possibly
interacting with associations46. Furthermore, MVMR could be subject to weak instrument bias75. A violation of the assumed linearity of causal effects could further bias the estimate76. The
results presented in this work provide several avenues of future research. The central limitation of statistical power has to be overcome with larger sample sizes or more accurate
measurements. Further research on risk factors mediating cancer risk and EA as exposure also hinges on that requirement. Another potential, more methodological avenue of research is
concerned with the addition of functional genetic variants to the developed pipeline. This would equip us with more tools to produce accurate estimates and validation thereof. Medical
science has rightly focused on how to treat people with cancer, however, any attempts to prevent the development of cancers are of utmost importance. Our finding, as well as work from
others, indicate that the number of years in education has a proportional impact on cancer. Although it is not possible for everyone to reach the same level of EA, this work identifies our
priorities in achieving similar benefits through targeted interventions. Currently, the detrimental effects of smoking and obesity are part of primary and secondary school curriculum in
several countries. Given their relatively recent inclusion though, it is still early to quantify their effectiveness, as cancer is more common in older individuals that left education before
the focus in healthier lifestyles. Nevertheless, our results suggest that we may see the gains of this strategy in the future and that a focus to educate children in primary and secondary
education on the dangers of smoking and how to better maintain a healthy weight should be adopted more widely. In addition, investigations should tailor interventions to accommodate people
that have already left education at secondary school level. Focusing our efforts of “good-health” education on the identified factors is likely to have the biggest impact on cancer rates.
DATA AVAILABILITY UK Biobank individual level data used in this work can be accessed after applying for access at https://www.ukbiobank.ac.uk/enable-your-research/apply-for-access. Genetic
association data are publicly available in the original studies. REFERENCES * Heron, M. & Anderson, R. N. Changes in the leading cause of death: Recent patterns in heart disease and
cancer mortality. _NCHS Data Brief._ 254, 1–8 (2016). Google Scholar * Gjertsen, F., Bruzzone, S. & Griffiths, C. E. Burden of suicide presented as one of the leading causes of death:
Uncover facts or misrepresent statistics?. _J. Glob. Health_ https://doi.org/10.7189/jogh.09.010401 (2019). Article PubMed Google Scholar * Sung, H. _et al._ Global cancer statistics
2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. _CA Cancer J. Clin._ 71(3), 209–249 (2021). Article PubMed Google Scholar * Leuven, E.,
Plug, E. & Ronning, M. Education and cancer risk. _Labour Econ._ 43, 106–121 (2016). Article Google Scholar * Siegel, R. L. _et al._ An assessment of progress in cancer control. _CA
Cancer J. Clin._ 68(5), 329–339 (2018). Article PubMed Google Scholar * Bray, F. _et al._ Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. _CA Cancer J. Clin._ 68(6), 394–424 (2018). Article PubMed Google Scholar * Bingham, S. & Riboli, E. Diet and cancer - The European prospective investigation
into cancer and nutrition. _Nat. Rev. Cancer_ 4(3), 206–215 (2004). Article CAS PubMed Google Scholar * Wilson, L., Bhatnagar, P. & Townsend, N. Comparing trends in mortality from
cardiovascular disease and cancer in the United Kingdom, 1983–2013: Joinpoint regression analysis. _Popul. Health Metr._ https://doi.org/10.1186/s12963-017-0141-5 (2017). Article PubMed
PubMed Central Google Scholar * Williams, D. R., Priest, N. & Anderson, N. B. Understanding associations among race, socioeconomic status, and health: Patterns and prospects. _Health
Psychol._ 35(4), 407–411 (2016). Article PubMed PubMed Central Google Scholar * Shohaimi, S. _et al._ Residential area deprivation predicts fruit and vegetable consumption independently
of individual educational level and occupational social class: A cross sectional population study in the Norfolk cohort of the European Prospective Investigation into Cancer (EPIC-Norfolk).
_J. Epidemiol. Commun. H_ 58(8), 686–691 (2004). Article Google Scholar * Teng, A. M., Atkinson, J., Disney, G., Wilson, N. & Blakely, T. Changing socioeconomic inequalities in cancer
incidence and mortality: Cohort study with 54 million person-years follow-up 1981–2011. _Int. J. Cancer_ 140(6), 1306–1316 (2017). Article CAS PubMed Google Scholar * Mirowsky, J. &
Ross, C. E. _Education, Social Status, and Health_ 1st edn. (Routledge, 2003). Google Scholar * Boylan, S. _et al._ Socio-economic circumstances and food habits in Eastern, Central and
Western European populations. _Public Health Nutr._ 14(4), 678–687 (2011). Article PubMed Google Scholar * Adler, N. E. _et al._ Socioeconomic status and health. The challenge of the
gradient. _Am. Psychol._ 49(1), 15–24 (1994). Article CAS PubMed Google Scholar * Warnakulasuriya, S. Significant oral cancer risk associated with low socioeconomic status. _Evid. Based
Dent._ 10(1), 4–5 (2009). Article PubMed Google Scholar * Geyer, S., Hemstrom, O., Peter, R. & Vagero, D. Education, income, and occupational class cannot be used interchangeably in
social epidemiology. Empirical evidence against a common practice. _J. Epidemiol. Community Health_ 60(9), 804–10 (2006). Article PubMed PubMed Central Google Scholar * Ovrum, A.
Socioeconomic status and lifestyle choices: Evidence from latent class analysis. _Health Econ._ 20(8), 971–984 (2011). Article PubMed Google Scholar * Nordahl, H. _et al._ Education and
cause-specific mortality: The mediating role of differential exposure and vulnerability to behavioral risk factors. _Epidemiology_ 25(3), 389–396 (2014). Article PubMed Google Scholar *
Gallo, V. _et al._ Social inequalities and mortality in Europe–results from a large multi-national cohort. _PLoS One_ 7(7), e39013 (2012). Article CAS PubMed PubMed Central ADS Google
Scholar * Hastert, T. A., Ruterbusch, J. J., Beresford, S. A., Sheppard, L. & White, E. Contribution of health behaviors to the association between area-level socioeconomic status and
cancer mortality. _Soc. Sci. Med._ 148, 52–58 (2016). Article PubMed Google Scholar * Lund Nilsen, T. I., Johnsen, R. & Vatten, L. J. Socio-economic and lifestyle factors associated
with the risk of prostate cancer. _Br. J. Cancer_ 82(7), 1358–1363 (2000). Article CAS PubMed Google Scholar * Conway, D. I. _et al._ Socioeconomic inequalities and oral cancer risk: A
systematic review and meta-analysis of case-control studies. _Int. J. Cancer_ 122(12), 2811–2819 (2008). Article CAS PubMed Google Scholar * Jepsen, P., Johnsen, S. P., Gillman, M. W.
& Sorensen, H. T. Interpretation of observational studies. _Heart_ 90(8), 956–960 (2004). Article CAS PubMed PubMed Central Google Scholar * Hernan, M. A. The hazards of hazard
ratios. _Epidemiology_ 21(1), 13–15 (2010). Article MathSciNet PubMed PubMed Central Google Scholar * Schuit, A. J., van Loon, A. J., Tijhuis, M. & Ocke, M. Clustering of lifestyle
risk factors in a general adult population. _Prev. Med._ 35(3), 219–224 (2002). Article PubMed Google Scholar * Smith, G. D. & Ebrahim, S. “Mendelian randomization”: Can genetic
epidemiology contribute to understanding environmental determinants of disease?. _Int. J. Epidemiol._ 32(1), 1–22 (2003). Article PubMed Google Scholar * Blakely, T., McKenzie, S. &
Carter, K. Misclassification of the mediator matters when estimating indirect effects. _J. Epidemiol. Community Health_ 67(5), 458–466 (2013). Article PubMed Google Scholar * Davies, N.
M., Holmes, M. V. & Davey, S. G. Reading Mendelian randomization studies: A guide, glossary, and checklist for clinicians. _BMJ_ 362, k601 (2018). Article PubMed PubMed Central Google
Scholar * Thanassoulis, G. & O’Donnell, C. J. Mendelian Randomization nature’s randomized trial in the post-genome era. _JAMA J. Am. Med. Assoc._ 301(22), 2386–2388 (2009). Article
CAS Google Scholar * Sudlow, C. _et al._ UK Biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. _PloS Med._ 12(3),
e1001779 (2015). Article PubMed PubMed Central Google Scholar * Bycroft, C. _et al._ The UK Biobank resource with deep phenotyping and genomic data. _Nature_ 562(7726), 203 (2018).
Article CAS PubMed PubMed Central ADS Google Scholar * Okbay, A. _et al._ Polygenic prediction of educational attainment within and between families from genome-wide association
analyses in 3 million individuals. _Nat. Genet._ 54(4), 437 (2022). Article CAS PubMed PubMed Central Google Scholar * Saunders, G. R. B. _et al._ Genetic diversity fuels gene discovery
for tobacco and alcohol use. _Nature_ 612(7941), 720 (2022). Article CAS PubMed PubMed Central ADS Google Scholar * Liu, M. Z. _et al._ Association studies of up to 1.2 million
individuals yield new insights into the genetic etiology of tobacco and alcohol use. _Nat. Genet._ 51(2), 237 (2019). Article CAS PubMed PubMed Central Google Scholar * Wootton, R. E.
_et al._ Evidence for causal effects of lifetime smoking on risk for depression and schizophrenia: A Mendelian randomization study. _Psychol. Med._ 50(14), 2435–2443 (2020). Article PubMed
Google Scholar * Leffondré, K., Abrahamowicz, M., Xiao, Y. L. & Siemiatycki, J. Modelling smoking history using a comprehensive smoking index: Application to lung cancer. _Stat. Med._
25(24), 4132–4146 (2006). Article MathSciNet PubMed Google Scholar * Yengo, L. _et al._ Meta-analysis of genome-wide association studies for height and body mass index in similar to
700,000 individuals of European ancestry. _Hum. Mol. Genet._ 27(20), 3641–3649 (2018). Article CAS PubMed PubMed Central Google Scholar * Elsworth B L, M, Alexander, T, Liu, Y,
Matthews, P, Hallett, J, Bates, P, Palmer, T, Haberland, V, Smith, G D, Zheng, J., Haycock, P, Gaunt, T R, Hemani, G. The MRC IEU OpenGWAS data infrastructure. BioRxiv (2020). * Genomes
Project C _et al._ An integrated map of genetic variation from 1092 human genomes. _Nature_ 491(7422), 56–65 (2012). Article ADS Google Scholar * Palmer, T. M. _et al._ Using multiple
genetic variants as instrumental variables for modifiable risk factors. _Stat. Methods Med. Res._ 21(3), 223–242 (2012). Article MathSciNet PubMed PubMed Central Google Scholar *
Burgess, S., Butterworth, A. & Thompson, S. G. Mendelian randomization analysis with multiple genetic variants using summarized data. _Genet. Epidemiol._ 37(7), 658–665 (2013). Article
PubMed PubMed Central Google Scholar * Bowden, J., Smith, G. D. & Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger
regression. _Int. J. Epidemiol._ 44(2), 512–525 (2015). Article PubMed PubMed Central Google Scholar * Bowden, J., Smith, G. D., Haycock, P. C. & Burgess, S. Consistent estimation in
mendelian randomization with some invalid instruments using a weighted median estimator. _Genet. Epidemiol._ 40(4), 304–314 (2016). Article PubMed PubMed Central Google Scholar *
Verbanck, M., Chen, C. Y., Neale, B. & Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and
diseases. _Nat. Genet._ 50(5), 693 (2018). Article CAS PubMed PubMed Central Google Scholar * Mounier, N. & Kutalik, Z. Bias correction for inverse variance weighting Mendelian
randomization. _Genet. Epidemiol._ 47(4), 314–331 (2023). Article CAS PubMed Google Scholar * Burgess, S., Dudbridge, F. & Thompson, S. G. Re: Multivariable Mendelian randomization:
The use of pleiotropic genetic variants to estimate causal effects. _Am. J. Epidemiol._ 181(4), 290–291 (2015). Article PubMed Google Scholar * Burgess, S., Daniel, R. M., Butterworth, A.
S. & Thompson, S. G. Network Mendelian randomization: Using genetic variants as instrumental variables to investigate mediation in causal pathways. _Int. J. Epidemiol._ 44(2), 484–95
(2015). Article PubMed Google Scholar * Sanderson, E. Multivariable Mendelian randomization and mediation. _CSH Perspect. Med._ https://doi.org/10.1101/cshperspect.a038984 (2021). Article
Google Scholar * RC T. R: A language and environment for statistical computing. R Foundation for Statistical Computing (2020). * Hemani, G. Z. J. _et al._ The MR-base platform supports
systematic causal inference across the human phenome. _elife_ https://doi.org/10.7554/eLife.34408 (2018). Article PubMed PubMed Central Google Scholar * Gordon M L, T, Gordon, M M. R
Package ‘forestplot’. Advanced Forest Plot Using ‘grid’ Graphics (2016). * Siegel, R., Ward, E., Brawley, O. & Jemal, A. Cancer statistics, 2011: The impact of eliminating socioeconomic
and racial disparities on premature cancer deaths. _CA Cancer J. Clin._ 61(4), 212–236 (2011). Article PubMed Google Scholar * Gornick, M. E., Eggers, P. W. & Riley, G. F.
Associations of race, education, and patterns of preventive service use with stage of cancer at time of diagnosis. _Health Serv. Res._ 39(5), 1403–1427 (2004). Article PubMed PubMed
Central Google Scholar * Mouw, T. _et al._ Education and risk of cancer in a large cohort of men and women in the United States. _PLoS One_ 3(11), e3639 (2008). Article PubMed PubMed
Central ADS Google Scholar * Cavelaars, A. E. _et al._ Educational differences in smoking: International comparison. _BMJ_ 320(7242), 1102–1107 (2000). Article CAS PubMed PubMed
Central Google Scholar * de Walque, D. Does education affect smoking behaviors? Evidence using the Vietnam draft as an instrument for college education. _J. Health Econ._ 26(5), 877–895
(2007). Article PubMed Google Scholar * He, J. B., Chen, X. J., Fan, X. T., Cai, Z. H. & Huang, F. Is there a relationship between body mass index and academic achievement? A
meta-analysis. _Public Health_ 167, 111–124 (2019). Article PubMed Google Scholar * De Irala-Estevez, J. _et al._ A systematic review of socio-economic differences in food habits in
Europe: Consumption of fruit and vegetables. _Eur. J. Clin. Nutr._ 54(9), 706–714 (2000). Article CAS PubMed Google Scholar * Cooke, L. J. _et al._ Demographic, familial and trait
predictors of fruit and vegetable consumption by pre-school children. _Public Health Nutr._ 7(2), 295–302 (2004). Article CAS PubMed Google Scholar * Rosoff, D. B. _et al._ Educational
attainment impacts drinking behaviors and risk for alcohol dependence: Results from a two-sample Mendelian randomization study with ~780,000 participants. _Mol. Psychiatr._ 26(4), 1119–1132
(2021). Article CAS Google Scholar * O’Keeffe, L. M. _et al._ Smoking as a risk factor for lung cancer in women and men: A systematic review and meta-analysis. _BMJ Open_ 8(10), e021611
(2018). Article PubMed PubMed Central Google Scholar * Minami, Y. & Tateno, H. Associations between cigarette smoking and the risk of four leading cancers in Miyagi Prefecture,
Japan: A multi-site case-control study. _Cancer Sci._ 94(6), 540–547 (2003). Article CAS PubMed Google Scholar * Pesch, B. _et al._ Cigarette smoking and lung cancer–relative risk
estimates for the major histological types from a pooled analysis of case-control studies. _Int. J. Cancer_ 131(5), 1210–1219 (2012). Article CAS PubMed Google Scholar * Larsson, S. C.
_et al._ Smoking, alcohol consumption, and cancer: A mendelian randomization study in UK Biobank and international genetic consortia participants. _PloS Med._ 17(7), e1003178 (2020). Article
PubMed PubMed Central Google Scholar * Scherubl, H. Excess body weight and gastrointestinal cancer risk. _Visc. Med._ 37(4), 261–266 (2021). Article PubMed PubMed Central Google
Scholar * Vithayathil, M. _et al._ Body size and composition and risk of site-specific cancers in the UK Biobank and large international consortia: A mendelian randomization study. _PloS
Med._ 18(7), e1003706 (2021). Article PubMed PubMed Central Google Scholar * Menvielle, G. _et al._ The role of smoking and diet in explaining educational inequalities in lung cancer
incidence. _J. Natl. Cancer Inst._ 101(5), 321–330 (2009). Article PubMed Google Scholar * Wang, C., Yang, T., Guo, X. F. & Li, D. The associations of fruit and vegetable intake with
lung cancer risk in participants with different smoking status: A meta-analysis of prospective cohort studies. _Nutrients_ 11(8), 1791 (2019). Article PubMed PubMed Central Google Scholar
* Vieira, A. R. _et al._ Fruits, vegetables and lung cancer risk: A systematic review and meta-analysis. _Ann. Oncol._ 27(1), 81–96 (2016). Article CAS PubMed Google Scholar * Wang, J.
_et al._ Citrus fruit intake and lung cancer risk: A meta-analysis of observational studies. _Pharmacol. Res._ 166, 105430 (2021). Article CAS PubMed Google Scholar * Fry, A. _et al._
Comparison of sociodemographic and health-related characteristics of UK biobank participants with those of the general population. _Am. J. Epidemiol._ 186(9), 1026–34 (2017). Article PubMed
PubMed Central Google Scholar * Park, J. H., Han, K., Hong, J. Y., Park, Y. S. & Park, J. O. Association between alcohol consumption and pancreatic cancer risk differs by glycaemic
status: A nationwide cohort study. _Eur. J. Cancer._ 163, 119–27 (2022). Article CAS PubMed Google Scholar * Zhang, X. Y. _et al._ Alcohol consumption and risk of cardiovascular disease,
cancer and mortality: a prospective cohort study. _Nutr. J._ 20(1), (2021). * Brion, M. J., Shakhbazov, K. & Visscher, P. M. Calculating statistical power in Mendelian randomization
studies. _Int. J. Epidemiol._ 42(5), 1497–1501 (2013). Article PubMed Google Scholar * Burgess, S. & Thompson, S. G. Bias in causal estimates from Mendelian randomization studies with
weak instruments. _Stat. Med._ 30(11), 1312–1323 (2011). Article MathSciNet PubMed Google Scholar * Rees, J. M. B., Wood, A. M. & Burgess, S. Extending the MR-Egger method for
multivariable Mendelian randomization to correct for both measured and unmeasured pleiotropy. _Stat. Med._ 36(29), 4705–4718 (2017). Article MathSciNet PubMed PubMed Central Google
Scholar Download references FUNDING The work was supported by a Brunel Research Initiative and Enterprise Fund to FD. The funders were not involved in the analysis and interpretation of the
data, in the writing of the report, or in the decision to submit the paper for publication. This research has been conducted using the UK Biobank Resource under project 44566
(https://www.ukbiobank.ac.uk/2018/12/genetic-and-non-genetic-factors-able-to-predict-and-modify-the-risk-of-different-types-of-cancer/). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS *
Department of Life Sciences, College of Health, Medicine and Life Sciences, Brunel University London, Kingston Lane, Uxbridge, London, UB8 3PH2, UK Loukas Zagkos, Hasnat A. Amin, Terry Dovey
& Fotios Drenos * Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, St Mary’s Campus, London, W2 1PG, UK Loukas Zagkos * Department of
Infectious Diseases, Faculty of Medicine, National Heart & Lung Institute, Imperial College London, Cale Street, London, SW3 6LY, UK Alexander Schwinges Authors * Loukas Zagkos View
author publications You can also search for this author inPubMed Google Scholar * Alexander Schwinges View author publications You can also search for this author inPubMed Google Scholar *
Hasnat A. Amin View author publications You can also search for this author inPubMed Google Scholar * Terry Dovey View author publications You can also search for this author inPubMed Google
Scholar * Fotios Drenos View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Conceptualization: F.D. and A.S., Funding acquisition: F.D.,
Formal Analysis: L.Z. and A.S., Data curation: L.Z., A.S. and H.A., Supervision: F.D. and T.D., Methodology: L.Z., A.S. and F.D., Writing – original draft: L.Z., A.S., F.D. and T.D., Writing
– review & editing: L.Z., A.S., H.A., F.D. and T.D. CORRESPONDING AUTHORS Correspondence to Loukas Zagkos or Fotios Drenos. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare
no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
SUPPLEMENTARY INFORMATION SUPPLEMENTARY TABLES. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use,
sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative
Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated
otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or
exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints
and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Zagkos, L., Schwinges, A., Amin, H.A. _et al._ Exploring the contribution of lifestyle to the impact of education on the risk of cancer
through Mendelian randomization analysis. _Sci Rep_ 14, 6074 (2024). https://doi.org/10.1038/s41598-024-54259-7 Download citation * Received: 08 March 2023 * Accepted: 10 February 2024 *
Published: 13 March 2024 * DOI: https://doi.org/10.1038/s41598-024-54259-7 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link
Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative