Identification of key autophagy-related genes and pathways in spinal cord injury
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Spinal cord injury (SCI) can cause a range of functional impairments, and patients with SCI have limited potential for functional recovery. Previous studies have demonstrated that
autophagy plays a role in the pathological process of SCI, but the specific mechanism of autophagy in this context remains unclear. Therefore, we explored the role of autophagy in SCI by
identifying key autophagy-related genes and pathways. This study utilized the GSE132242 expression profile dataset, which consists of four control samples and four SCI samples;
autophagy-related genes were sourced from GeneCards. R software was used to screen differentially expressed genes (DEGs) in the GSE132242 dataset, which were then intersected with
autophagy-related genes to identify autophagy-related DEGs in SCI. Subsequently, the expression levels of these genes were confirmed and analyzed with gene ontology (GO) and the Kyoto
Encyclopedia of Genes and Genomes (KEGG). A protein–protein interaction (PPI) analysis was conducted to identify interaction genes, and the resulting network was visualized with Cytoscape.
The MCODE plug-in was used to build gene cluster modules, and the cytoHubba plug-in was applied to screen for hub genes. Finally, the GSE5296 dataset was used to verify the reliability of
the hub genes. We screened 129 autophagy-related DEGs, including 126 up-regulated and 3 down-regulated genes. GO and KEGG pathway enrichment analysis showed that these 129 genes were mainly
involved in the process of cell apoptosis, angiogenesis, IL-1 production, and inflammatory reactions, the TNF signaling pathway and the p53 signaling pathway. PPI identified 10 hub genes,
including CCL2, TGFB1, PTGS2, FN1, HGF, MYC, IGF1, CD44, CXCR4, and SERPINEL1. The GSE5296 dataset revealed that the control group exhibited lower expression levels than the SCI group,
although only CD44 and TGFB1 showed significant differences. This study identified 129 autophagy-related genes that might play a role in SCI. CD44 and TGFB1 were identified as potentially
important genes in the autophagy process after SCI. These findings provide new targets for future research and offer new perspectives on the pathogenesis of SCI. SIMILAR CONTENT BEING VIEWED
BY OTHERS IDENTIFICATION AND BIOINFORMATICS ANALYSIS OF GENES ASSOCIATED WITH PYROPTOSIS IN SPINAL CORD INJURY OF RAT AND MOUSE Article Open access 18 June 2024 UNVEILING VITAL BIOMARKERS
AND IMMUNE INFILTRATION PROFILES IN ENDOPLASMIC RETICULUM STRESS FOLLOWING SPINAL CORD INJURY Article Open access 02 December 2024 INTEGRATED BIOINFORMATICS ANALYSIS IDENTIFIED
CUPROPTOSIS-RELATED HUB GENE MPEG1 AS POTENTIAL BIOMARKER IN SPINAL CORD INJURY Article Open access 15 January 2025 INTRODUCTION Spinal cord injury (SCI) is a devastating neurological injury
that can cause motor sensory dysfunction and severely impact a patient's quality of life. Estimates of the global incidence of SCI range greatly, from approximately 3 in 1,000,000 to 2
in 10,0001. SCI is characterized by two pathological stages: primary injury and secondary injury2. Primary injury refers to the irreversible damage caused by a direct physical injury,
resulting in neuronal and cell death in the spinal cord tissue and the disruption of the blood–brain barrier. Secondary injury, which occurs alongside primary injury, is characterized by
tissue edema, inflammatory reactions, neuron apoptosis, glial scar formation, and sphingomyelin loss3. Patients with SCI face extreme physical and psychological trauma, and the expensive
treatment and nursing costs place a large burden on patients, their families, and society. SCI is considered one of the top priorities in global health problems, but no completely effective
treatment methods have been identified. Therefore, exploring the molecular mechanism of SCI pathophysiology is crucial. Autophagy is a self-degradation and recycling process that is highly
conserved and coordinated4. It mainly occurs in eukaryotic cells and can be categorized as macro-autophagy, micro-autophagy, or chaperone-mediated autophagy5. Autophagy separates misfolded
proteins, damaged organelles, and other substances and fuses them into lysosomes, resulting in their degradation6. Autophagy is highly activated in the early stage of SCI, but its specific
role in SCI is still debated. Some studies have reported that autophagy can inhibit cell death and apoptosis after SCI, promoting functional recovery, whereas others suggest that autophagy
plays a role in neuronal death after SCI7,8,9. Considering these conflicting results, further exploration of the specific mechanism of autophagy activation in SCI is crucial. Despite the
growing body of research on the role of autophagy in SCI, few studies have analyzed key genes and pathways using bioinformatics methods. Examining the expression of autophagy-related genes
in spinal cord tissue after SCI can help elucidate the molecular mechanism of autophagy. By analyzing the GSE132242 dataset in the Gene Expression Omnibus (GEO) database with bioinformatics
technology, we identified key genes and potential pathways related to autophagy after SCI, providing new targets for the diagnosis and treatment of this condition. MATERIALS AND METHODS DATA
COLLECTION AND ANALYSIS This study searched the GEO databas. The GSE132242 dataset was used as the test dataset, and the GSE5296 dataset was used as the validation dataset. The GSE132242
dataset comprised four SCI mouse samples and four spinal cord tissue samples from a sham operation group. The GSE5296 dataset comprised three SCI model samples and three spinal cord tissue
samples from a sham operation group. DATA PREPROCESSING AND SCREENING OF DIFFERENTIALLY EXPRESSED GENES (DEGS) For the GSE5296 dataset, we used the R software package "affy" to
normalize and log2 transform the downloaded raw data. For the GSE132242 dataset, the software package “getGEO” was used to acquire the expression data and platform file and we take the
average of the same genes. Next, we employed the R software package "limma" to identify the DEGs between the SCI and control samples, with significance thresholds of adjusted P
< 0.05 and │log2FC|> 1. Finally, we utilized the online tool "sangerbox" (http://www.sangerbox.com/login.html) to generate the heatmap cluster and volcano plots for the DEGs.
ACQUISITION OF AUTOPHAGY-RELATED GENES GeneCards is an extensive searchable database of genes that provides information about nearly all known genes (https://www.genecards.org/). We
downloaded all genes related to autophagy from the database, calculated the median of all genes based on the "score," and then identified genes that exceeded twice the median for
inclusion in our follow-up study. DIFFERENTIAL EXPRESSION ANALYSIS OF AUTOPHAGY-RELATED GENES Autophagy-related DEGs were identified as genes that overlapped between the DEGs and
autophagy-related genes. Next, the number of overlapping genes was visualized with a Venn diagram created using web tools (http://bioinformatics.psb.ugent.be/webtools/Venn/), and the
"ggplot2" package was used to generate a heatmap. CORRELATION ANALYSIS OF AUTOPHAGY-RELATED DEGS The Spearman correlation coefficient in the "corrplot" R package was used
to determine the correlation between autophagy-related DEGs in SCI. GENE ONTOLOGY (GO) AND KYOTO ENCYCLOPEDIA OF GENES AND GENOME (KEGG) ANALYSES OF AUTOPHAGY-RELATED DEGS IN SCI The GO
function is divided into three categories: biological processes (BP), cellular components (CC), and molecular functions (MF), while the KEGG pathways explain the primary functions of genes
at the molecular level. The "cluster profiler" and "GOplot" R software packages were used to perform GO and KEGG pathway enrichment analyses and visualizations for all
autophagy-related genes, and the adjusted P-value, was considered the threshold for significant enrichment. The results of the top 10 enriched categories were visualized with bubble charts
and bar charts. CONSTRUCTION OF THE PROTEIN–PROTEIN INTERACTION (PPI) NETWORK AND IDENTIFICATION OF CENTRAL GENES AND KEY MODULES To elucidate the functional interactions between proteins,
the Search Tool for Recurring Instances of Neighbouring Genes (STRING, https://cn.string-db.org/) and Cytoscape software (https://cytoscape.org/) were utilized. Specifically, the
"Betweenness" algorithm in the "CytoNCA" plug-in was used to analyze all genes and construct a circular PPI network based on the "Betweenness" score.
Subsequently, the "MCODE" plug-in was used to cluster the gene network and identify key sub-network modules. Lastly, the "CytoHubb" plug-in was employed to screen the top
10 hub genes using a variety of built-in algorithms. STATISTICAL ANALYSIS In this study, the SPSS 26.0 version was utilized for statistical analysis. First, the Shapiro–Wilk method was
applied to test whether the data conformed to a normal distribution. For data conforming to a normal distribution, we employed an independent sample Student's t-test to compare the gene
expression levels of samples. For non-normal distribution data, we used the Mann–Whitney U test. _P_-value less than 0.05 was considered statistically significant. RESULTS DIFFERENTIALLY
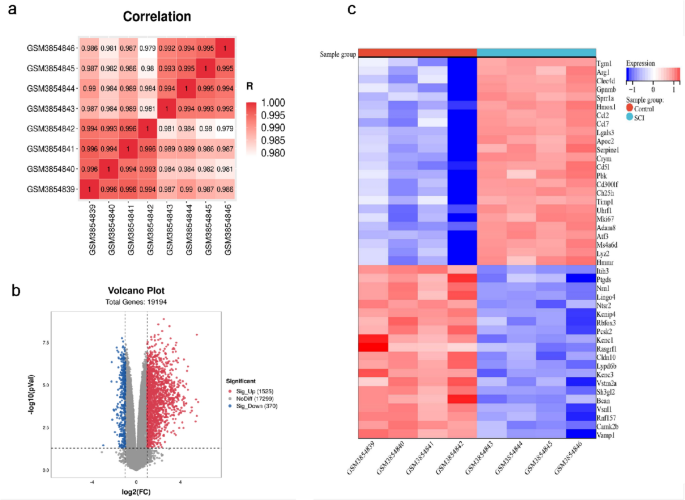
EXPRESSED GENES PROFILES IN SCI Initially, we conducted correlation analysis on four SCI samples and four control samples, and the results indicated that the sample quality was reliable
(Fig. 1a). Then, we screened 1,895 DEGs from a total of 19,194 genes. Of these, 1525 genes were up-regulated, and 370 genes were down-regulated. The findings were illustrated in the volcano
map (Fig. 1b). Finally, the up-regulated and down-regulated genes were extracted and visualized with heatmaps (Fig. 1c). DIFFERENTIAL EXPRESSION ANALYSIS OF AUTOPHAGY-RELATED GENES Using the
two-fold median standard, we identified 1293 autophagy-related genes from the GeneCards gene database. Subsequently, we screened 129 autophagy-related DEGs, including 126 up-regulated and 3
down-regulated genes (Fig. 2a–c). CORRELATION ANALYSIS OF AUTOPHAGY-RELATED DEGS The screening criteria for autophagy-related DEGs were P < 0.05 and │log2FC|> 1. Autophagy-related
DEGs were ranked according to the value of │log2FC |, and the greater the value of │log2FC|, the greater the trend of gene difference. We performed a correlation analysis on the top 50 of
the 129 autophagy-related DEGs. The results revealed different correlations among the 50 genes (Fig. 3). Notably, FAS and CSTB showed the strongest positive correlation (Cor = 1.00).
Additionally, several negatively correlated gene pairs were identified among the 50 genes, including IL-24 and PCNA, VAMP8 and PNF1, and TUBA8 and IGFBP3, of these pairs, IL-24 and CD44
having the strongest negative correlation (Cor = − 0.98). GO AND KEGG ANALYSES OF AUTOPHAGY-RELATED DEGS IN SCI To determine the biological processes and KEGG pathways of the 129
autophagy-related DEGs, we conducted GO annotation and KEGG enrichment analysis using the "clusterProfiler" R software package. We identified 674 significantly enriched GO
biological process terms and selected the top 10 terms from BP, CC, and MF for visualization. The BP terms were mainly associated with the apical processes, positive regulation of gene
expression, and angiogenesis. The CC terms were mainly enriched in the cytoplasm, nucleus, and cytosol. By contrast, the MF terms were mainly enriched in protein binding, authentic protein
binding, and macromolecular complex binding (Fig. 4a,b, Tables 1, 2, and 3). In the KEGG enrichment analysis, the 129 autophagy-related DEGs were significantly enriched in 93 KEGG pathway
terms, including the TNF signaling pathway, apoptosis, and the p53 signaling pathway (Fig. 4c,d, Table 4). CONSTRUCTION OF THE PPI NETWORK AND IDENTIFICATION OF CENTRAL GENES AND KEY MODULES
To investigate the interactions between the 129 autophagy-related DEGs, we performed a PPI analysis using the STRING database and visualized the results with Cytoscape. First, we used the
"CytoNCA" plug-in to build a PPI network based on the Betweenness score (Fig. 5a). In addition, we used the MCODE plug-in to identify important gene cluster modules and identified
three clusters. Cluster 1 contained 39 nodes and 271 edges, with a score of 14.263. Cluster 2 contained 21 nodes and 73 edges, with a score of 7.300, while cluster 3 contained seven nodes
and 10 edges, with a score of 3.333 (Fig. 5b–d). To identify central genes, we used the CytoHubba plug-in, which identified 10 central genes: CCL2, TGFB1, PTGS2, FN1, HGF, MYC, IGF1, CD44,
CXCR4, and SERPINE1 (Fig. 5e). CROSS-VALIDATION OF EXTERNAL DATASETS To verify the accuracy of our results, we conducted cross-validation with the GSE5296 dataset to examine the expression
levels of the 10 key genes. We downloaded this dataset from the GEO database and selected samples with the same damage time as those in the GSE132242 dataset for analysis. Except for
SERPINE1, the expression levels of the nine remaining key genes were similar to those in the GSE132242 dataset. Specifically, the control group exhibited lower expression levels than the SCI
group, although only CD44 and TGFB1 showed significant differences. The expression levels of the remaining seven key genes did not show a significant difference between the two groups (Fig.
6). DISCUSSION The results of the GO analysis revealed that the 129 autophagy-related DEGs mainly functioned in the cytoplasm, nucleus, and cytosol. Their molecular functions were related
to protein binding and macromolecular complex binding. These genes were involved in apoptosis, angiogenesis, and the regulation of IL-1β production and the inflammatory response.
Additionally, the KEGG enrichment analysis demonstrated that these genes were mainly enriched in the TNF signaling pathway, apoptosis, and the p53 signaling pathway. The TNF and the p53
signaling pathways are mainly associated with the neuroinflammatory reaction and the apoptosis of cells and neurons in SCI. These series of analysis results confirmed that autophagy played a
crucial role in SCI by regulating inflammatory response and apoptosis and might also impact angiogenesis. p53 is a crucial regulator of apoptosis, and many apoptosis-related molecules exert
their effects through p53, resulting in a complex process10. A prior study has identified p53 and Bax-dependent cell apoptosis induced by DNA damage as the main cause of spinal motor neuron
death after nerve avulsion11. Another study has reported that p53-mediated spinal cord mitochondrial apoptosis induced by DNA damage is an essential mechanism of cell death after SCI12.
Moreover, p53 is involved in cell survival and axon growth, indicating that it is a critical factor influencing functional recovery after SCI and plays a vital regulatory role in neurite
outgrowth13. Additionally, SIRT1 may also inhibit SCI cell apoptosis by regulating the p53 signaling pathway14. In recent years, as research on Traditional Chinese Medicine has expanded,
scholars have discovered that Schisandrin B can reduce the inflammatory response, oxidative stress, and apoptosis of in SCI by inhibiting the p53 signaling pathway15. Similarly, Buyang
Huanwu Decoction treats SCI by regulating the p53 signaling pathway16. These findings support the results of our study, indicating that the p53 signaling pathway plays an essential role in
SCI. This study found that IGF1 was enriched in the p53 signaling pathway. Previous research has demonstrated that IGF1 inhibited autophagy by activating the PI3K/Akt/mTOR signaling pathway,
which promoted functional recovery after SCI in rats. However, it is unclear whether IGF1 regulates autophagy through the p53 signaling pathway and contributes to the pathogenesis of SCI;
therefore, further investigation is necessary17. It is well-established that TNF plays a pivotal role in the inflammatory response following SCI by inducing cytokine and chemokine
expression18. Another study has observed that the TNF signaling pathway remained activated throughout the course of SCI, with stronger activation during the early stages19. On the first day
after the injury, a combination of TNF, recombinant IL-6, and IL-1 at the lesion site led to the recruitment and activation of microglia and macrophages. However, by the fourth day, TNF
administration reduced the activation of microglia and the size of the lesion area, suggesting that TNF plays different roles at different time points after SCI20. This study found that two
hub genes (CCL2 and PTGS2) were found to be enriched in the TNF signaling pathway. CCL2 is an important chemokine that regulates autophagy and responds to various physiological and
pathophysiological stimuli by activating autophagy. The inhibition of CCL2 expression and the PI3K/Akt/mTOR signaling pathway can activate autophagy, effectively reducing neuronal apoptosis
after SCI21. In another study, macrophage migration inhibitory factor inhibitors improved the motor function of rats' hind limbs by reducing the microglia and macrophages recruited by
CCL2 at the injury site22. Recent studies based on bioinformatics analysis have demonstrated that PTGS2 is related to iron death and immune infiltration after SCI23,24. Our research suggests
that PTGS2 affects the functional recovery of mice after SCI by regulating autophagy, but the specific mechanism of action requires further investigation. Moreover, the GO and PPI analyses
revealed that several genes might contribute significantly to the pathophysiology of SCI. For example, autophagy-related DEGs identified through GO analysis could regulate the production of
IL-1β, the main mediator of inflammation, which plays a harmful role in SCI. Inhibiting IL-1β can have protective effects in SCI25. The up-regulation of CD44 after SCI contributes to cell
adhesion and glial cell attraction, promoting SCI injury repair26. The SDF-1/CXCR4 interaction recruits exogenous mesenchymal stem cells into injured spinal cord tissue, which may enhance
nerve regeneration. Furthermore, the CXCR4 signaling pathway is involved in the migration of Schwann cells from the peripheral nervous system to the central nervous system after SCI,
improving motor function27,28. HGF is endogenously produced in the spinal cord of rats after SCI29 and gradually increases during the first week after injury, remaining at a high level in
the short term. HGF helps reduce the extent of SCI and improve functional recovery by exhibiting anti-inflammatory, anti-apoptotic, angiogenic, anti-fibrotic, and neurogenic properties in
transplanted neural stem cells (NSCs)30. TGF-β is significantly upregulated by microglia and macrophages at the epicenter, rostral, and caudal areas after SCI and plays a crucial role in
regulating nerve regeneration31. It modulates neurite growth, promotes glial scar formation, and interacts with immune cells to mediate inflammation and the immune response induced by nerve
injury32. The formation of the glial scar is attributed to chondroitin sulfate proteoglycan (CSPG). Up-regulated TGF-β after SCI can inhibit the autophagic flux, enhance the secretion of
CSPG, and impair nerve regeneration. Targeted inhibition of TGF-β can restore the autophagic flux, reduce the formation of the glial scar, and the recovery of spinal cord function33. This
study has several limitations that need to be acknowledged. First, we only used the latest dataset for our analysis, resulting in a limited sample size and possible deviations in our
results. Second, the validation dataset was published in July 2006, and errors due to technical reasons are unavoidable. Additionally, different SCI operation methods may lead to different
results. Third, further study of the potential mechanism of the selected hub genes is limited because of a lack of in vivo and in vitro experiments. CONCLUSION We screened 129
autophagy-related DEGs through bioinformatics analysis and identified vital pathways related to these genes. Additionally, we identified 10 hub genes including CCL2, TGFB1, PTGS2, FN1, HGF,
MYC, IGF1, CD44, CXCR4, and SERPINE1. After multiple validations, the results suggested that CD44 and TGFB1 as potential research and treatment targets for autophagy after SCI. The follow-up
study will experimentally verify the results of this study. DATA AVAILABILITY The datasets used and/or analysed during the current study available from the corresponding author on
reasonable request. REFERENCES * Ashammakhi, N. _et al._ Regenerative therapies for spinal cord injury. _Tissue Eng. Part B Rev._ 25(6), 471–491. https://doi.org/10.1089/ten.TEB.2019.0182
(2019). Article PubMed PubMed Central Google Scholar * Alizadeh, A., Dyck, S. M. & Karimi-Abdolrezaee, S. Traumatic spinal cord injury: An overview of pathophysiology, models and
acute injury mechanisms. _Front. Neurol._ 10, 282. https://doi.org/10.3389/fneur.2019.00282 (2019). Article PubMed PubMed Central Google Scholar * Sekhon, L. H. & Fehlings, M. G.
Epidemiology, demographics, and pathophysiology of acute spinal cord injury. _Spine._ 26(24 Suppl), S2-12. https://doi.org/10.1097/00007632-200112151-00002 (2001). Article CAS PubMed
Google Scholar * Avin-Wittenberg, T. Autophagy and its role in plant abiotic stress management. _Plant Cell Environ._ 42(3), 1045–1053. https://doi.org/10.1111/pce.13404 (2019). Article
CAS PubMed Google Scholar * Mizushima, N. & Komatsu, M. Autophagy: Renovation of cells and tissues. _Cell._ 147(4), 728–741. https://doi.org/10.1016/j.cell.2011.10.026 (2011). Article
CAS PubMed Google Scholar * Behrends, C., Sowa, M. E., Gygi, S. P. & Harper, J. W. Network organization of the human autophagy system. _Nature._ 466(7302), 68–76.
https://doi.org/10.1038/nature09204 (2010). Article ADS CAS PubMed PubMed Central Google Scholar * Zhang, H. _et al._ 3,4-Dimethoxychalcone, a caloric restriction mimetic, enhances
TFEB-mediated autophagy and alleviates pyroptosis and necroptosis after spinal cord injury. _Theranostics._ 13(2), 810–832. https://doi.org/10.7150/thno.78370 (2023). Article CAS PubMed
PubMed Central Google Scholar * Zhang, H. _et al._ Piperine attenuates the inflammation, oxidative stress, and pyroptosis to facilitate recovery from spinal cord injury via autophagy
enhancement. _Phytother. Res. PTR._ 37(2), 438–451. https://doi.org/10.1002/ptr.7625 (2023). Article CAS PubMed Google Scholar * Chen, H. C., Fong, T. H., Lee, A. W. & Chiu, W. T.
Autophagy is activated in injured neurons and inhibited by methylprednisolone after experimental spinal cord injury. _Spine._ 37(6), 470–475. https://doi.org/10.1097/BRS.0b013e318221e859
(2012). Article CAS PubMed Google Scholar * Saito, N., Yamamoto, T., Watanabe, T., Abe, Y. & Kumagai, T. Implications of p53 protein expression in experimental spinal cord injury.
_J. Neurotrauma._ 17(2), 173–182. https://doi.org/10.1089/neu.2000.17.173 (2000). Article CAS PubMed Google Scholar * Martin, L. J. & Liu, Z. Injury-induced spinal motor neuron
apoptosis is preceded by DNA single-strand breaks and is p53- and Bax-dependent. _J. Neurobiol._ 50(3), 181–197. https://doi.org/10.1002/neu.10026 (2002). Article CAS PubMed Google
Scholar * Kotipatruni, R. R. _et al._ p53- and Bax-mediated apoptosis in injured rat spinal cord. _Neurochem. Res._ 36(11), 2063–2074. https://doi.org/10.1007/s11064-011-0530-2 (2011).
Article CAS PubMed Google Scholar * Floriddia, E. M. _et al._ p53 Regulates the neuronal intrinsic and extrinsic responses affecting the recovery of motor function following spinal cord
injury. _J. Neurosci._ 32(40), 13956–13970. https://doi.org/10.1523/jneurosci.1925-12.2012 (2012). Article CAS PubMed PubMed Central Google Scholar * Yu, X. _et al._ SIRT1 inhibits
apoptosis in in vivo and in vitro models of spinal cord injury via microRNA-494. _Int. J. Mol. Med._ 43(4), 1758–1768. https://doi.org/10.3892/ijmm.2019.4106 (2019). Article CAS PubMed
PubMed Central Google Scholar * Xin, D. Q. _et al._ Schisandrin B attenuates the inflammatory response, oxidative stress and apoptosis induced by traumatic spinal cord injury via
inhibition of p53 signaling in adult rats. _Mol. Med. Rep._ 16(1), 533–538. https://doi.org/10.3892/mmr.2017.6622 (2017). Article CAS PubMed PubMed Central Google Scholar * Wang, Y.,
Chen, H., Wang, J., Chen, X. & Chen, L. Exploring the mechanism of Buyang Huanwu Decoction in the treatment of spinal cord injury based on network pharmacology and molecular docking.
_Medicine._ 101(40), 31023. https://doi.org/10.1097/md.0000000000031023 (2022). Article Google Scholar * Zhang, D. _et al._ Insulin-like growth factor 1 promotes neurological functional
recovery after spinal cord injury through inhibition of autophagy via the PI3K/Akt/mTOR signaling pathway. _Exp. Ther. Med._ 22(5), 1265. https://doi.org/10.3892/etm.2021.10700 (2021).
Article CAS PubMed PubMed Central Google Scholar * Esposito, E. & Cuzzocrea, S. Anti-TNF therapy in the injured spinal cord. _Trends Pharmacol. Sci._ 32(2), 107–115.
https://doi.org/10.1016/j.tips.2010.11.009 (2011). Article CAS PubMed Google Scholar * Gong, L. _et al._ Changes in transcriptome profiling during the acute/subacute phases of
contusional spinal cord injury in rats. _Ann. Transl. Med._ 8(24), 1682. https://doi.org/10.21037/atm-20-6519 (2020). Article CAS PubMed PubMed Central Google Scholar * Klusman, I.
& Schwab, M. E. Effects of pro-inflammatory cytokines in experimental spinal cord injury. _Brain Res._ 762(1–2), 173–184. https://doi.org/10.1016/s0006-8993(97)00381-8 (1997). Article
CAS PubMed Google Scholar * Fang, S. _et al._ Identification of the CCL2 PI3K/Akt axis involved in autophagy and apoptosis after spinal cord injury. _Metab. Brain Dis._ 1, 1.
https://doi.org/10.1007/s11011-023-01181-y (2023). Article CAS Google Scholar * Zhang, H. _et al._ Macrophage migration inhibitory factor facilitates astrocytic production of the CCL2
chemokine following spinal cord injury. _Neural Regen.Res._ 18(8), 1802–1808. https://doi.org/10.4103/1673-5374.363184 (2023). Article CAS PubMed Google Scholar * Li, J. Z. _et al._
Bioinformatics analysis of ferroptosis in spinal cord injury. _Neural Regen. Res._ 18(3), 626–633. https://doi.org/10.4103/1673-5374.350209 (2023). Article CAS PubMed Google Scholar *
YANG, S. _et al._ Bioinformatics-based diagnosis and evaluation of several pivotal genes and pathways associated with immune infiltration at different time points in spinal cord injury.
_biotechnol. Genet. Eng. Rev._ 1, 1–27. https://doi.org/10.1080/02648725.2023.2178970 (2023). Article CAS Google Scholar * Chen, Y. Q. _et al._ CRID3, a blocker of apoptosis associated
speck like protein containing a card, ameliorates murine spinal cord injury by improving local immune microenvironment. _J. Neuroinflam._ 17(1), 255.
https://doi.org/10.1186/s12974-020-01937-8 (2020). Article CAS Google Scholar * Moon, C., Heo, S., Sim, K. B. & Shin, T. Upregulation of CD44 expression in the spinal cords of rats
with clip compression injury. _Neurosci. Lett._ 367(1), 133–136. https://doi.org/10.1016/j.neulet.2004.05.101 (2004). Article CAS PubMed Google Scholar * Zhao, A. _et al._ The
SDF-1/CXCR4 signaling pathway directs the migration of systemically transplanted bone marrow mesenchymal stem cells towards the lesion site in a rat model of spinal cord injury. _Curr. Stem
Cell Res. Ther._ 18(2), 216–230. https://doi.org/10.2174/1574888x17666220510163245 (2023). Article CAS PubMed Google Scholar * Furumiya, T., Itokazu, T., Nakanishi, T. & Yamashita,
T. CXCR4 signaling regulates repair Schwann cell infiltration into the spinal cord after spinal cord injury in mice. _Neurosci. Res._ 1, 1. https://doi.org/10.1016/j.neures.2022.12.022
(2022). Article CAS Google Scholar * Kitamura, K. _et al._ Hepatocyte growth factor promotes endogenous repair and functional recovery after spinal cord injury. _J. Neurosci. Res._
85(11), 2332–2342. https://doi.org/10.1002/jnr.21372 (2007). Article CAS PubMed Google Scholar * Yamane, K. _et al._ Multipotent neurotrophic effects of hepatocyte growth factor in
spinal cord injury. _Int. J. Mol. Sci._ 20(23), 1. https://doi.org/10.3390/ijms20236078 (2019). Article CAS Google Scholar * McTigue, D. M., Popovich, P. G., Morgan, T. E. & Stokes,
B. T. Localization of transforming growth factor-beta1 and receptor mRNA after experimental spinal cord injury. _Exp. Neurol._ 163(1), 220–230. https://doi.org/10.1006/exnr.2000.7372 (2000).
Article CAS PubMed Google Scholar * Li, S., Gu, X. & Yi, S. The regulatory effects of transforming growth factor-β on nerve regeneration. _Cell Transp._ 26(3), 381–394.
https://doi.org/10.3727/096368916x693824 (2017). Article Google Scholar * Alizadeh, J. _et al._ Inhibition of autophagy flux promotes secretion of chondroitin sulfate proteoglycans in
primary rat astrocytes. _Mol. Neurobiol._ 58(12), 6077–6091. https://doi.org/10.1007/s12035-021-02533-4 (2021). Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS We
thank TopEdit (www.topeditsci.com) for its linguistic assistance during the preparation of this manuscript. FUNDING This work was supported by Natural Science Foundation of China (grant
number: 31872310). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Medical Department of Qingdao University, Qingdao, 266000, China Zhen Shang & Weipeng Shi * Department of Orthopedic
Surgery, The Affiliated Hospital of Qingdao University, Qingdao, 266000, Shandong, China Haitao Fu & Yingze Zhang * Shandong Institute of Traumatic Orthopedics, Qingdao, 266000, China
Yingze Zhang * Department of Orthopedic Surgery, Qingdao Municipal Hospital, Qingdao, 266000, China Tengbo Yu * Institute of Sports Medicine and Health, Qingdao University, Qingdao, 266000,
China Tengbo Yu * Department of Orthopedic Surgery, Qingdao Hospital, University of Health and Rehabilitation Sciences, Qingdao, 266000, China Tengbo Yu Authors * Zhen Shang View author
publications You can also search for this author inPubMed Google Scholar * Weipeng Shi View author publications You can also search for this author inPubMed Google Scholar * Haitao Fu View
author publications You can also search for this author inPubMed Google Scholar * Yingze Zhang View author publications You can also search for this author inPubMed Google Scholar * Tengbo
Yu View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Tengbo Yu and Yingze Zhang contributed to the conception and design of this study. Zhen
Shang, Weipeng Shi and Haitao Fu conducted preliminary data analysis and collation. Zhen Shang, Weipeng Shi, Haitao Fu, Tengbo Yu and Yingze Zhang conducted further data analysis and wrote
the manuscript. All authors have read and approved the final manuscript. CORRESPONDING AUTHORS Correspondence to Yingze Zhang or Tengbo Yu. ETHICS DECLARATIONS COMPETING INTERESTS The
authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional
affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution
and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if
changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the
material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will
need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE
CITE THIS ARTICLE Shang, Z., Shi, W., Fu, H. _et al._ Identification of key autophagy-related genes and pathways in spinal cord injury. _Sci Rep_ 14, 6553 (2024).
https://doi.org/10.1038/s41598-024-56683-1 Download citation * Received: 09 November 2023 * Accepted: 09 March 2024 * Published: 19 March 2024 * DOI:
https://doi.org/10.1038/s41598-024-56683-1 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * Spinal cord injury * Autophagy * Bioinformatics
analysis * Therapeutic target