Tissue-specific accumulation of PIP aquaporins of a particular heteromeric composition is part of the maize response to mycorrhiza and drought
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

The systemic coordination of accumulation of plasma membrane aquaporins (PIP) was investigated in this study in relation to mycorrhized maize response to a rapid development of severe
drought followed by rewatering. In non-mycorrhizal roots, drought led to a drop in PIP abundance, followed by a transient increase under rewatering, whereas leaves showed an opposite
pattern. In contrast, mycorrhiza contributed to maintenance of high and stable levels of PIPs in both plant organs after an initial increase, prolonged over the irrigation period.
Isoelectric focusing electrophoresis resolved up to 13 aquaporin complexes with highly reproducible pl positions across leaf and root samples, symbiotic and non-symbiotic, stressed or not.
Mass spectrometry recognized in leaves and roots a different ratio of PIP1 and PIP2 subunits within 2D spots that accumulated the most. Regardless of symbiotic status, drought regulation of
aquaporins in roots was manifested as the prevalence of complexes that comprise almost exclusively PIP2 monomers. In contrast, the leaf response involved enrichment in PIP1s. PIP1s are
thought to enhance water transport, facilitate CO2 diffusion but also affect stomatal movements. These features, together with elevated aquaporin levels, might explain a stress tolerance
mechanism observed in mycorrhizal plants, resulting in faster recovery of stomatal water conductance and CO2 assimilation rate after drought.
Arbuscular mycorrhizal (AM) symbiosis occurs between soil fungi—representing the division Glomeromycota—and a large majority of vascular land plants. The host plant improves water and
nutrient uptake thanks to an extensive network of mycelium that both penetrates the root cortex cell layer and can reach soil zones inaccessible to the roots1,2.
AM symbiosis improves host performance under a variety of abiotic stresses, such as drought or salinity3,4,5,6. An often suggested explanation for this effect is improved plant nutrition;
however, mycorrhizal fungi can modify host water relations in a way entirely unrelated to increased acquisition of mineral compounds7. Published meta-analysis data provide evidence that
extraradical water uptake is an equally important fungal benefit that can influence plant fitness8. Therefore, it is postulated to untangle experimentally these two alternative components,
to identify more directly the factors that promote the hydraulic conductance of roots, leaf gas exchange parameters, and thus maintenance of the root-leaf water potential gradient. In
addition to the effect on plant stress-related genes, AM symbiosis modifies the expression of aquaporins7,9,10,11, which seems to be under the control of complex hormonal
signalization12,13,14.
Plasma membrane aquaporins (PIP proteins) are tetrameric water channels that maintain transmembrane water permeability, providing an alternative to apoplastic lateral water
distribution15,16. Transport of xylem water is separated from the apoplastic radial pathway by the hydrophobic barrier of the Casparian strips in roots and the tightly packed ring of bundle
sheath cells that envelop the vascular system in leaves15,17. Cell-to-cell water flow through aquaporins and plasmodesmata is one of the postulated mechanisms that allows water to pass these
‘control zones’. Therefore, one of the proposed hypotheses explaining the limitations of long-distance water transport and transpiration involves controlling radial water flow by regulation
of aquaporin expression and activity17,18,19,20.
On this account, when discussing issues related to water availability in mycorrhizal interactions, the level of functionality of plant (and fungal) aquaporins becomes an issue of central
importance10,21,22,23,24. It seems that the synergistic interaction of fungal and plant aquaporins at the interface between the cell membrane and arbuscular structures is the most effective
model of regulation, thus contributing to symbiotic drought tolerance9,25.
The activity of plant aquaporins may be substantially influenced by the proportion of isoforms that make up their tetrameric structure. Oligomerization occurs through the interaction of
α-helices of neighbouring monomers and an extracellular A loop, which contributes to the stabilization of the tetramer26,27,28.
Plant PIP proteins can be divided into two large groups, PIP1 and PIP2, based on their sequence and water channel activity. PIP2 homotetramers show high water channel activity when expressed
in Xenopus oocytes or in yeast. PIP1 homotetramers produced in such systems are usually inactive or have very low activity; however, this effect may be eliminated by coexpression with PIP2
homologues29,30. This confirmed the hypothesis that heterotetramer formation may play an important role in the correct location of water channels on the surface of plant cells31. This rule
also applies to maize (Zea mays), where an increase in permeability coefficient has been shown to occur if ZmPIP1;2 is coexpressed with different ZmPIP2 isoforms30.
Interestingly, coexpression of PIP1s and PIP2s results in increased membrane permeability, greater than when only PIP2-PIP2 homocomplexes are formed30,32. The tissue colocalization of the
PIP1-PIP2 transcripts and their similar abundance pattern in response to different stress conditions support the hypothesis that the interaction between these proteins is a key mechanism
enabling plants to maintain the water status. The generation of the associations of PIP1s and PIP2s must be regulated at the transcriptional and post-translational levels. However,
transcriptional variation affects the abundance of proteins, which consequently determines the ratio of PIP1 to PIP2 present in the cell membrane, thus dictating the functionality of PIP
tetramers32.
Published data on the expression of plant PIP proteins show that the PIP1 and PIP2 isoforms are always present together in plasma membranes. Protein immunoprecipitation experiments have
provided evidence for the physical interactions of ZmPIP1;2 and ZmPIP2;1 also in maize roots and cell suspensions31. Differences in the expression level of each member of the subfamily
determine the appearance of functional PIP1-PIP2 or PIP2-PIP2 tetramers32,33,34,35. However, the stoichiometry within heterocomplexes consisting of proteins of each group may differ
significantly in tissues from distant organs, such as roots and leaves. Therefore, studies are needed to reveal aquaporin interactions in plant cells and to determine the physiological
relevance of these processes, also in the context of mycorrhizal cooperation.
In our previous study36, mycorrhizal (AM) plants showed a much faster reversal of drought-induced leaf senescence, stomatal water conductance, and CO2 fixation rate than their
non-mycorrhizal (NM) counterparts. In the present study we test the hypothesis that rapid recovery of AM maize from deep water deficits is linked to symbiotic regulation of maize aquaporins.
In the following, we argue that the ratio of PIP1 and PIP2 monomers that form aquaporin heterocomplexes, corresponds to the patterns of their tissue-specific accumulation under given
hydration conditions and their abundance can be altered by the presence of mycorrhiza.
Plant cultures (Zea mays L., hybrid Opoka, Plant Breeding Smolice Ltd., Poland) and seedling inoculation with Rhizophagus irregularis spores, obtained from monoxenic root cultures37 were
carried out in a high fertilized soil-free semi hydroponic system according to the procedure described previously36 until silking (63 BBCH stage, 12 weeks after sowing). The plant culture
was carried out in such a way to ensure that long-term mycorrhizae vitality remained undisturbed and that symbiotic plants did not differ from non-mycorrhized counterparts in terms of shoot
size and plant nutritional status. At silking time-point the plants were subjected to fast (as compared to our previous research36) and reversible water-stress procedure described below. All
research under this study was conducted according to Polish national law and did not require any additional permits.
We designed a rapid development of water stress to minimize leaf senescence progression related to drought-altered nutrients availability. Well-watered plants were removed from pots and
transferred, leaving the root system exposed, to the cabinet with low air humidity (30%). The mixture of coconut fiber and sand in which the roots remained immersed helped to obtain severe
but reversible drought effects in as little as 7 days. Soil drought was imposed by stopping irrigation for 7 days to achieve a severe drop in plant water potential ( leaves < -1.5 MPa),
followed by renewed fertilizer irrigation for 5 days until complete rehydration of plant tissues.
Before stress imposition, both symbiotic variants did not differ significantly in nitrogen and phosphorus content within each leaf shoot position investigated. This was due to the
high-fertilized cultivation system used, discussed in our previous work36, in which the symbiotic state does not affect the growth and the nutritional status of the plants (Supplementary
Fig. 1). Using this cultivation setup, the level of mycorrhizal colonization was achieved in the present study at the similar level as previously, reaching stable, more than 55%, arbuscular
abundance in colonized parts of root fragments at the time of experiments (12 weeks after sowing, not shown).
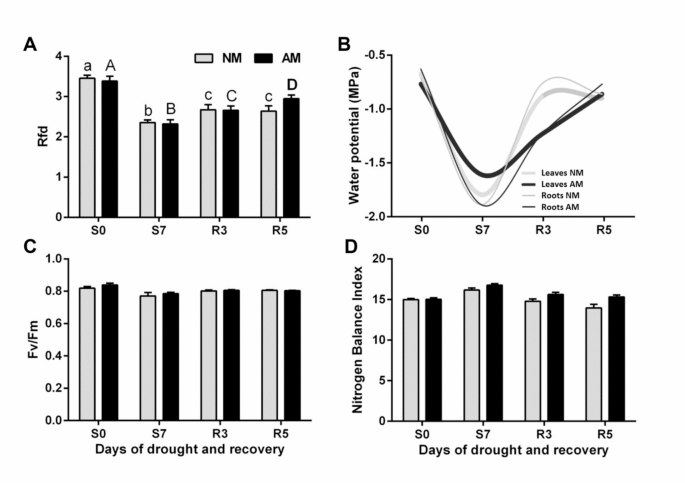
The midday leaf and root water potential, the light-saturated leaf gas exchange capacity (stomatal water conductance; gs, transpiration rate, Tmax and photosynthetic rate; Amax), chlorophyll
fluorescence kinetics (fluorescence decrease ratio, Rfd and maximum PSII quantum yield in a dark-adapted state, Fv/Fm) and leaf nitrogen management index (NBI) were measured as described
in36. The evaluation of leaf physiology was carried out in middle leaves (the ear leaf and the leaf above the cob).
The samples were taken from combined middle leaves (ear leaf and leaf above), or the mix of secondary feeder roots (diameter less than 1.5 mm), collected from four plants removed on
consecutive days of water treatment. A modification of the methods of Abas and Luschnig38 and Santoni39 was developed: 5 g of plant tissue was homogenized with 12.5 ml of buffer: 100 mM
Tris-HCl pH 7,5, 25% saccharose (w/w), 5% glycerol (v/v), 10 mM EDTA pH 8, 10 mM EGTA pH 8, 5 mM KCl, 1 mM DTT, 0,2% casein, 1% PIC (Protease Inhibitor Cocktail, Merck) and the sediment of
buffer-saturated polyvinylpolypyrrolidone. The homogenate was filtered through Miracloth (Millipore) and centrifuged for 10 min at 10,000 g at 4 °C. The supernatant was diluted with water to
obtain 12–13% saccharose at final volume. After centrifugation at 100,000 g for 1 h at 4 °C, the microsomal sediment was washed, centrifuged again, and resuspended in the buffer: 20 mM Tris
HCl pH 7.5, 5 mM EDTA, 5 mM EGTA, 1 mM PMSF, 1% PIC. Protein concentration was determined using the Bradford40 method.
SDS-PAGE electrophoresis was performed on 11% polyacrylamide gels according to the TGX Stain-Free method (BioRad), with addition of 4 M urea. 10 µg of protein samples were mixed in a 1:1,6
ratio (v/v) with solubilization buffer (96 mM Tris-HCl pH 6.8, 9.6 M urea, 24% saccharose, 3.2% SDS, 5% β-mercaptohetanol), and denatured at 60 °C for 10 min. The semidry blotter system
(Merck) was then used to transfer proteins to Immobilon-P membrane, according to Millipore instructions.
Immunochemical identification was carried out with anti-PIP1;1–3 (Agrisera AS09 489) and anti-PIP2;1–7 (Agrisera, AS12 2110) antibodies. The membrane was blocked overnight at 4 °C in 3% BSA
in PBS and then incubated for 1 h in RT with PIP1;1–3 antibody at 1:1000 dilution and with PIP2;1–7 antibody at 1:3000 dilution in PBS-T buffer containing 1% BSA. The membrane was then
incubated for 1 h in RT with the secondary antibodies (Agrisera, AS09 6), at a 1:20000 dilution in 1 x PBS-T. Antigens were detected with Lumi-Light Western Blotting Kit (Roche).
For estimation of PIP monomers composition, the spots were taken from untreated gels, according to coordinates determined by immunodetection on parallel gels, and then subjected to tandem
mass spectrometry procedure.
The microsomes were treated with Brij-58 detergent to delipidate and remove surface-associated proteins41. 120 µg of protein, 240 µl 2% Brij-58 and 4 µl PIC were gently mixed for 30 min at 4
°C. After overnight precipitation at -26 °C with 10% (w/v) TCA in acetone and 0.07% (v/v) β-mercaptoethanol the proteins were pelleted for 15 min at 20,000 g and then washed three times
with pure acetone and 0,07% β-mercaptohetanol. Then, acetone was removed and the precipitate was dried for 10 min. The solubilization of integral membrane proteins was carried out by
suspending 120 µg of protein in 120 µl of IEF buffer containing: 7 M urea, 2 M thiourea, 2% ASB-14, 65 mM DTT, 1% PIC and 1% IPG 3–10 buffer (Bio-Rad).
Isoelectrofocusing was carried out at 20 °C in PROTEAN 12TM IEF Cell (BioRad) using IPG strips with immobilized 3–10 pH gradient (BioRad), first for 1.5 h at 300 V, then for 1.5 h in the
300–3500 V gradient and next at 3500 V until 20,000 Vh was reached. After IEF, the strips were equilibrated according to our modification of the McDonough and Marbán42 method. IPG strips
were placed for 15 min in 10 ml of pH equilibrating buffer: 65 mM DTT, 6 M urea, 30% glycerol, 6% SDS, 50 mM Tris-HCl pH 6,8 and then alkylated for 15 min in a buffer containing 2,5%
iodoacetamide instead of 65 mM DTT. For protein separation according to their molecular masses, the strips were subjected to Stain-Free SDS-PAGE as above.
To identify physical interactions of aquaporin subunits, proteins were isolated by immunoprecipitation, according to our modification of the method described in Zelazny, et al.31. 250 µg of
microsomes were solubilized by adding 2% ASB-14 and 0.1% TX-100 detergents suspended in 300 µl of TBS in the presence of 1.7% PIC. The sample was incubated for 2 h at 4 °C with stirring and
then centrifuged for 10 min at 110,000 g. The supernatant was collected and diluted 1:1 with TBS to obtain the final 1% concentration of ASB detergent. The samples were agitated overnight at
4 °C with 1 µL of PIP2;1–7 antibodies. Antigen-antibody complexes were bound to Protein A-Sepharose 4B bed (Merck), washed and then eluted by incubation with 1.6x sample buffer (20mM Tris
pH 6.8, urea 6 M, SDS 2%) at 60 °C for 10 min. The supernatant after the following centrifugation was quenched with 2 ml of cold acetone with 0.07% β-mercaptohetanol and precipitated as
described above. The obtained pellet was dried in a fume hood for 30 min and then dissolved in 100 µl buffer: 4 M urea, 2 M thiourea, 2% ASB-14, 65 mM DTT. The final acetone concentration
was 7 M to avoid the risk of precipitation of urea present in the sample buffer.
Protein spots were excised from the gel under sterile conditions and analysed by liquid chromatography (Nano-Acquity, Waters) coupled to the mass spectrometer (Orbitrap Velos or Orbitrap
Elite) in the Laboratory of Mass Spectrometry, Institute of Biochemistry and Biophysics, Polish Academy of Sciences (Warsaw, Poland) as previously described by Kubala, et al.43.
Raw data files were pre-processed with Mascot Distiller software (version 2.5, MatrixScience). The data obtained was compared with the database of data deposited in the amino acid sequences
of green plant proteins or Z. mays deposited in the NCBI database using the Mascot program (Mascot Server 2.4.1, Matrixscience). The following parameters were used for comparison: enzymatic
specificity: trypsin or semitrypsin, precision of mass measurement: ± 30 ppm, precision of the measurement accuracy: ± 0.1 Da, allowed number of deficiencies: 1, modification of amino acid
residues: oxidation, phosphorylation, carbamidomethylation. The basis for acceptance of the peptides was the score value threshold calculated by the Mascot program44. The mass spectrometry
proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE45 partner repository with the dataset identifier PXD046819 and https://doi.org/10.6019/PXD046819.
The abundance of MS identified PIP1 and PIP2 isoforms were drawn from Exponentially Modified Protein Abundance Index (emPAI) - the index estimating protein abundances from peptide counts in
a single LC-MS/MS experiment, implemented within MASCOT software. EmPAI is defined as 10PAI minus one, where PAI has been defined as the ratio of the number of observed peptides to the
number of observable peptides. This is a strictly probabilistic method however based on empirical approximations from the large-scale proteome profiling experiments46,47.
The experimental data are presented as means of a specified number of replicates (n) ± SEM. Statistical analysis was carried out using the Student’s t-test, for groups with normal
distribution (Shapiro-Wilk test), one-way or two-way analysis of variance (ANOVA) and Tukey-Kramer multiple comparison test. Statistically significant results were those for which the level
of statistical significance value (p) reached: p ≤ 0,05 (*), p