The critical role of dimethylamine in the rapid formation of iodic acid particles in marine areas
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Marine new particle formation (NPF) can affect cloud condensation nuclei (CCN) in the global atmosphere. Recently, iodic acid (IA) has been identified as a critical driver for
marine NPF. However, atmospheric observations of IA cannot be associated with predicted particle formation rates. Given the complexity of atmospheric components, other species may promote IA
particle formation. As an efficient stabilizer for acidic precursors, dimethylamine (DMA) has a wide distribution over the oceans. Hence, we investigated the nucleation process of DMA and
IA under different atmospheric conditions and uncovered the corresponding nucleating mechanism using a quantum chemical approach and Atmospheric Cluster Dynamics Code (ACDC). The findings
show that DMA can structurally stabilize IA via hydrogen and halogen bonds, and the clustering process is energy barrierless. Moreover, DMA can enhance the formation rate of IA clusters by
five orders of magnitude, and its efficiency in promoting IA cluster formation is much higher than that of NH3. Compared to the nucleation via sequential addition of IA, the IA-DMA
nucleation plays a more dominant role in nucleation kinetic. Thus, the effect of DMA on enhancing IA cluster stability and formation rate cannot be ignored, especially in the regions near
the source of IA and DMA emissions. Broadly, the proposed IA-DMA nucleation mechanism may help to explain some missing sources of particles and, thus intensive marine NPF events. SIMILAR
CONTENT BEING VIEWED BY OTHERS OPEN OCEAN AND COASTAL NEW PARTICLE FORMATION FROM SULFURIC ACID AND AMINES AROUND THE ANTARCTIC PENINSULA Article 13 May 2021 SIGNIFICANT CONTRIBUTIONS OF
TRIMETHYLAMINE TO SULFURIC ACID NUCLEATION IN POLLUTED ENVIRONMENTS Article Open access 27 June 2023 GLOBAL VARIABILITY IN ATMOSPHERIC NEW PARTICLE FORMATION MECHANISMS Article Open access
12 June 2024 INTRODUCTION Marine aerosol acts as the most important natural aerosol system in the world, significantly affecting global radiation balance and climate system through further
conversion to cloud condensation nuclei (CCN)1,2. New particle formation (NPF) initiated via the nucleating of gaseous molecules provides an important source of atmospheric aerosol3,4.
Hence, to better understand the marine aerosol formation, the critical challenge involves the chemical speciation of nucleation precursors and disentangling the corresponding nucleating
mechanism at the molecular level5,6. Globally, marine NPF are significantly influenced by biogenic emissions, although anthropogenic impacts are also involved, to a lesser extent, especially
in the remote ocean7. Field observations have shown that marine NPF is closely related to the iodine-bearing molecules8, which originate from iodine vapors emitted by marine algal9,10.
Among these iodine components, iodic acid (HIO3, IA) has been identified to be the key NPF driver in the coastal, open ocean, and ice-covered polar regions11,12,13. However, atmospheric
observations of IA cannot be associated with predicted particle formation rates14. Given the complexity of atmospheric components, other widespread precursors over the oceans, especially
from biological emissions, may also be involved in the formation process of IA particles. Amines (e.g., monomethylamine MA, dimethylamine DMA, and trimethylamine TMA), as nitrogenous bases
widely distributed in the atmosphere15, possess relatively strong basicity and consequently stabilization effect on acidic nucleation precursors such as well-known sulfuric acid (SA)15,16
and methanesulfonic acid (MSA)17,18. As the stronger stabilizer of these amine molecules (DMA ≥ TMA > MA)16, the most extensively studied DMA has been identified as a major nucleating
precursor in the coastal city of Shanghai, China19, coastal California20, and over the open ocean21,22. Moreover, the widespread DMA has a considerable atmospheric concentration (0.4–10
pptv) over the ocean, making it important for marine NPF23. Considering the ability of DMA to efficiently stabilize acidic precursors and its wide distribution in the marine
atmosphere24,25,26,27, there is a potential for DMA to stabilize IA molecules via acid-base reactions. However, whether DMA is capable of rapid clustering with IA and the corresponding
nucleation process are unknown at the molecular level. To investigate the nucleation process of IA and DMA, the combination of a quantum chemical method with Atmospheric Clusters Dynamic
Code (ACDC)28 were employed to simulate the nucleation kinetic process. A multi-step conformational search was systematically performed to identify stable conformations of (IA)_x_.(DMA)y (1
≤ _x_ ≤ 6, 1 ≤ _y_ ≤ 3, _x_ + _y_ ≤ 6) clusters with global minima energy. The nature of IA binding with DMA within the cluster was studied by wavefunction analysis, and cluster
thermodynamic properties are calculated to assess the stability of clusters. To further study IA-DMA nucleation under different marine areas, a series of ACDC simulations were carried out at
the corresponding atmospheric conditions (varying precursor concentrations and temperatures). Furthermore, to characterize the role of DMA in stabilizing IA clusters, the corresponding
molecular-level nucleating mechanism was further proposed by tracking the clustering pathways. RESULTS CLUSTER CONFORMATION Strong intermolecular interactions are essential for the formation
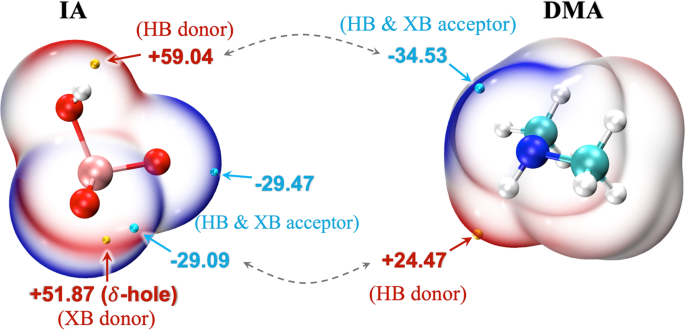
of stable molecular clusters. To evaluate the binding potential between IA and DMA, their possible interaction sites were first analyzed through the electrostatic surface potential (ESP) in
Fig. 1, where the red electron-deficient region tends to attract the blue electron-rich region. As illustrated in Fig. 1, IA has a positive ESP maximum (+59.04 kcal mol−1) at the H atom of
its -OH group, which can act as a hydrogen bond (HB) donor. And there is an ESP minimum (−29.09 kcal mol−1) at the terminal oxygen atom in IA, mainly attributed to its lone pair electrons,
making it available as an HB acceptor. As to DMA, the -NH group can serve as both HB donor and acceptor. In this case, hydrogen bonding between IA and DMA results in the formation of a
heterodimer (IA)1.(DMA)1 (Fig. 2). In addition to HB, IA also potentially forms halogen bonds (XB) with DMA or itself via its XB donor site (_δ_-hole with ESP value of +51.87 kcal mol−1)
with the -NH group in DMA (XB acceptor). Figure 2 presents the identified (IA)_x_.(DMA)_y_ (1 ≤ _x_ ≤ 6, 1 ≤ _y_ ≤ 3, 2 ≤ _x_ + _y_ ≤ 6) clusters with lowest free energies. Within these
clusters, IA and DMA are structurally stabilized by the network built with HBs (black dashed lines) and XBs (green dashed lines). Moreover, the acid-base reactions occurred during cluster
formation, accompanied by the proton transferring from IA to DMA. Among these IA-DMA clusters, proton transfer between IA and DMA occurred within all clusters except for the (IA)1-2.(DMA)3
clusters, and the binding sites of the unprotonated DMA were all involved in forming HBs or XBs. Specifically, HBs within IA-DMA clusters exist in four forms, in the order of number: N-H…O
(79%) > O-H…O (13%) > O-H…N (5%) > N-H…N (3%). The high percentage of N-H…O is due to the N-H covalent bonds formed by proton transfer (O-H…N → O…H-N) within most IA-DMA clusters.
For HB strength, according to the AIM calculation (Supplementary Table 1), the Laplacian electron density \(\nabla\)2_ρ_(_r_) at the BCPs is in the range of 0.0469–0.1689 (a.u.), and energy
density _H_(_r_) is −0.0250–0.0023 (a.u.). Further, based on the classification of HBs29, most of the formed HBs (79%) belong to medium HBs (12.0 < binding energy < 24.0 kcal mol−1)
with \(\nabla\)2_ρ_(_r_) > 0 and _H_(_r_) < 0. And for XBs within clusters, O-I…O contributes more (84%) than O-I…N XBs (16%), acting mainly between IA molecules. Overall, IA-DMA
clusters are jointly stabilized by multiple types of intermolecular HBs and XBs. Also, during the clustering process, the acid-base reactions between IA and DMA occur in most clusters,
resulting in acid-base ion pairs. CLUSTER STABILITY To further evaluate the thermodynamic stability of clusters, the Gibbs formation free energies (∆_G_) of the studied clusters were
calculated at the condition of _T_ = 218–298 K and _p_ = 1 atm (Supplementary Tables 2, 3). Figure 3a presents the ∆_G_ at 278 K of (IA)_x_.(DMA)_y_ (0 ≤ _x_ ≤ 6, 0 ≤ _y_ ≤ 3, 1 ≤ _x_ + _y_
≤ 6) clusters. Compared with pure (IA)2-6 clusters, the (IA)1-5.(DMA)1 clusters possess 8.01–16.56 kcal mol−1 lower ∆_G_s, indicating greater cluster thermodynamic stability enhanced by DMA.
Moreover, for IA-DMA clusters with the same number of molecules, the ∆_G_ of the cluster with more acid and less base is significantly lower than that of the cluster with more base and less
acid. Furthermore, the stabilization effect of DMA on IA was compared with that of another important atmospheric nitrogenous base molecule, NH3, as shown in Fig. 3. Taken as a whole, the
∆_G_s of IA-DMA clusters are 8.04–27.08 kcal mol−1 lower than those of the corresponding IA-NH3 clusters. Moreover, the total evaporation rates (∑_γ_ in s−1 at 278 K) of most IA-DMA clusters
presented in Fig. 3b are also lower than those of IA-NH3 clusters, except for the (IA)1-2.(DMA)3 clusters, probably due to the steric hindrance effect caused by three unprotonated DMA
(Supplementary Fig. 1). The lower ∑_γ_ and higher _βC_/∑_γ_ (Supplementary Fig. 2) of IA-DMA clusters further reflect their stronger cluster stability than IA-NH3 clusters. The detailed
cluster evaporation paths and the corresponding _γ_ at 278 K are collected in Supplementary Table 4. Furthermore, considering the difference in atmospheric concentrations of DMA and NH3, the
actual Gibbs free energy affected by vapor concentration30 was calculated by Eq. (3) and shown in Fig. 3c. For the pure-IA system (blue line), the clustering process by sequential addition
of IA monomer needs to cross an energy barrier of 10.59 kcal mol−1 at conditions of [IA] = 108 molecules cm−3. As to the IA-NH3 system (yellow line), the addition of NH3 can reduce the
energy barrier to 6.46 kcal mol−1 at [NH3] = 400 pptv (median value)31. While for the IA-DMA system, the proposed IA-DMA clustering path (red lines), in which the (IA)2-4.(DMA)2 and
(IA)1-5.(DMA)1 clusters dominate, is a barrierless process even at its lower concentration ([DMA] = 0.4 pptv)23. Overall, the IA-DMA clustering pathway is more energetically favorable than
the pure-IA and IA-NH3 systems (Supplementary Fig. 3), and the formed IA-DMA clusters have greater stability. CLUSTER FORMATION RATE To further study the IA-DMA nucleation kinetics, the
steady-state cluster formation rate _J_ (cm−3 s−1) were simulated by ACDC at ambient conditions of _T_ = 278 K and condensation sink coefficient (CS) of 2.0 × 10−3 s−1 for coastal region32.
In the simulations, the employed IA monomer concentration ([IA]) here is the total concentration of clusters consisting of one IA and any number of base, corresponding to the measurable acid
concentration. Figure 4 presents _J_ of the pure-IA (blue line), IA-NH3 (yellow area), and IA-DMA systems (red area) at the corresponding precursor concentrations over the oceans: [IA] =
106~108 (molecules cm−3)12, [DMA] = 0.01~10 pptv23,33 and [NH3] = 40~103 pptv31,34. As shown in Fig. 4, compared with pure-IA nucleation, the involvement of both DMA and NH3 in clustering
significantly promote the rate _J_, indicating that both have an enhancement effect on the formation of IA clusters. Interestingly, the far lower [DMA] (0.01~10 pptv) relative to [NH3]
(40~103 pptv) can promote _J_ to a higher level (red area), indicating that the formation of IA-involved particles is more sensitive to the enhancement effects of DMA. It is worth noting
that the enhancement of DMA on _J_ increases with [DMA], but dulling occurs for [DMA] greater than 1 pptv (Supplementary Fig. 7). Although the adopted [DMA] is three orders of magnitude
lower than [NH3], the resulting _J_ of IA-DMA system is still higher than that of IA-NH3. Further comparison (Supplementary Fig. 8) reveals that the _J_ of IA-DMA nucleation can better match
the field observations in Mace Head12 and Zhejiang8. Accordingly, the proposed efficient IA-DMA nucleation may help to explain some intensive marine NPF events with rapid IA cluster
formation. CLUSTER FORMATION PATHWAY To further reveal the mechanism of nucleation kinetics, the cluster formation pathways of the IA-DMA system were tracked at the molecular level by ACDC
(Fig. 5a). Kinetic simulations were performed under the conditions of _T_ = 278 K, CS = 2 × 10−3 s−1, [IA] = 108 (molecules cm−3)12, and [DMA] = 0.01~10 pptv23,33. As shown in Fig. 5a, the
cluster formation pathways can be divided into two categories: (i) the pure-IA self-nucleation and (ii) IA-DMA-based cluster formation. For the pure-IA pathway, the cluster growth proceeds
mainly via the sequential addition of IA monomer, which is consistent with the findings of the previous study35. And for the IA-DMA pathway proposed in this study, DMA first binds with IA to
form a heterodimer (IA)1.(DMA)1. Next, the formed (IA)1.(DMA)1 cluster continues growing by the addition of IA to form (IA)2.(DMA)1 cluster. The (IA)2.(DMA)1 cluster further collides with
DMA monomer and (IA)1.(DMA)1 to form (IA)2.(DMA)2 and (IA)3.(DMA)2 clusters, respectively. Subsequently, the cluster growth is mainly through the continuous addition of IA monomers,
resulting in larger (IA)3-4.(DMA)2 and (IA)4-5.(DMA)1 clusters. Finally, the formed (IA)3-4.(DMA)2 and (IA)5.(DMA)1 can further bind with IA monomer or IA-containing cluster to grow out of
the simulated system. Under different conditions, as shown in Fig. 5b, different out-growing paths contribute differently to _J_. At low [DMA] (0.01 pptv), flux out from (IA)5.(DMA)1
dominates (65%). And at [DMA] of 0.1~1.0 pptv, the cluster growth based on (IA)4.(DMA)2 contributes the most (72%). And when [DMA] increases to 10 pptv, cluster formation is mainly affected
by the formation of (IA)3.(DMA)2 clusters colliding with other clusters. Moreover, to assess the contribution of each cluster to the particle formation, the steady-state number
concentrations of all studied clusters were calculated at _T_ = 278 K, [IA] = 108 molecules and [DMA] = 0.4 pptv. As shown in Fig. 5c, the concentration of most DMA-containing clusters (blue
cycle), such as (IA)1-5.(DMA)1 and (IA)2-4.(DMA)2, is higher than that of pure-IA clusters (red cycle), due to the greater stability of IA-DMA clusters (recall Section 3.2). For the total
particle concentration, the IA-DMA nucleating pathway (blue line along (IA)1-5.(DMA)1 and (IA)2-4.(DMA)2 clusters) contributes more than that of the IA self-nucleation (red line along
(IA)2-6 clusters). In the largest clusters (IA)4.(DMA)2, (IA)5.(DMA)1, and (IA)6 with outgrowth potential (Supplementary Methods), the ratios of IA to DMA were calculated in Supplementary
Fig. 9 by weighting their steady-state concentrations at varying [IA] (106~ 108 molecules cm−3) and [DMA] (0.01~10 pptv). The resulting ratio of IA/DMA in these clusters is 2.0~ 3.5,
indicating that IA is still the main cluster component (67–78%). Overall, the IA-DMA clusters contribute more to the nucleating process than the pure-IA clusters. Although DMA is a minor
component of the formed IA-DMA cluster, it does play a key role in enhancing IA cluster formation. Accordingly, these stable IA-DMA clusters may provide a large number of seeds for the
marine NPF. ATMOSPHERIC IMPACT OF IA-DMA SYSTEM To quantify the promotion of DMA on IA cluster formation, we thus defined the enhancement strength as _R_ in Eq. (1): $$R = \frac{{J\left(
{[{{{\mathrm{IA}}}}] = x,[{{{\mathrm{DMA}}}}] = y} \right)}}{{J\left( {[{{{\mathrm{IA}}}}] = x} \right)}}$$ (1) where _x_ and _y_ are the gaseous concentrations of IA and DMA, respectively.
Atmospheric conditions vary with regions and seasons, which in turn sway the influence of the IA-DMA mechanism. Herein, as shown in Fig. 6, the enhancement of DMA on IA cluster formation was
simulated in different marine regions with the corresponding ambient conditions. In the ACDC simulation, the average temperature data for each month (orange lines) at different locations
was taken from the NASA database36, the [IA] were set with reference to the reported field observations14, and the [DMA] were set based on the GEOS-Chem simulations33, where the specific
values at different sites and moths were determined by the latitude and longitude of the studied sites. For mid-latitude oceans, CS is set to be a typical value (2.0 × 10−3 s−1) of coastal
regions32 for Mace Head, 1.0 × 10−2 s−1 for Zhejiang8, and for other high-latitude polar regions, CS is set to be 1.0 × 10−4 (s−1)14. The results indicate that _R_ is positively correlated
with the temperature (_T_) at each site. As shown in Fig. 6a–c, _R_ is higher during the summer months in the northern and southern hemispheres. Specifically, in coastal areas of the
northern hemisphere, such as Mace head, Zhejiang, and Ny-Ålesund, the enhancement _R_ of DMA on IA cluster formation (Green pillars) is relatively stronger in June–August. While in the
southern hemisphere offshore regions, such as Réunion, Aboa, and Neumayer (Fig. 6d–f), the higher _R_ occurs in November–March. The reason for this phenomenon is that _J_ of the pure-IA
system (denominator of _R_) is more sensitive to _T_ than that of the IA-DMA system (the numerator of _R_) (Supplementary Fig. 6). In addition to _T_, varying [IA] and [DMA] also affect _R_
to a greater or lesser extent. From the definition of enhancement _R_ (Eq. (1)), the [IA] affects both numerator (_J_IA-DMA) and denominator (_J_pure-IA) simultaneously, and thus has a
relatively small effect on their ratio (_R_). In contrast, although [DMA] has a greater effect on _R_, as it only affects the _J_IA-DMA (the numerator of _R_), it still does not present any
correlation with _R_. In summary, the effects of the IA-DMA mechanism vary with the ambient conditions of different marine regions. It shows a clear seasonal feature mainly influenced by
temperature all year round, specifically the enhancement of DMA on IA cluster formation rate can be more than five orders of magnitude, which is stronger in summer. This enhancement effect
of DMA is relatively stronger in the northern hemisphere oceans, due to the relatively richer DMA, than in the southern hemisphere. DISCUSSION Iodic acid (IA) has been identified as an
essential driver for marine NPF. As an essential stabilizer of atmospheric acidic precursors, dimethylamine (DMA) potentially stabilizes IA clusters via acid-base reactions. Hence, in this
study, we have investigated the (IA)_x_(DMA)y (1 ≤ _x_ ≤6, 1 ≤ _y_ ≤3, x + y ≤ 6) nucleating system using the density functional theory (DFT) and Atmospheric Cluster Dynamics Code (ACDC).
Results indicate that DMA can stabilize IA via hydrogen and halogen bonding, and the clustering process is energy barrierless. The formed IA-DMA clusters are more stable than pure-IA and
IA-NH3 clusters. Moreover, DMA can significantly promote the formation of IA clusters stemming from the resulting boosted _J_, especially in summer, and its promotion efficiency is much
higher than that of NH3. In the revealed IA-DMA nucleating pathways, the clustering process is dominated by the more stable IA-DMA clusters rather than the sequential addition of IA.
Overall, DMA potentially plays a critical role in enhancing IA cluster stability and, thus formation rates. The uncovered IA-DMA nucleation mechanism may help to reveal some missing sources
of fresh particles and thus better understand intensive marine NPF events. Considering the relatively short lifetime of DMA, the IA-DMA nucleation is especially effective in a hot-spot NPF
event near their emission sources. Considering the complexity of the marine atmosphere, many factors might affect the nucleation process of IA and DMA, such as the humidity effect. The
effect of water was not considered here because of its weak ability to bind IA37 and the hydrogen bonds formed by water are weaker than those between acid and base precursors38.
Nevertheless, the humidity effect on the nucleating process in regions with sparse IA and DMA still needs to be considered in future. In addition, the influence of other important nucleation
precursors such as sulfuric acid (H2SO4), methanesulfonic acid (CH4O3S), or iodine oxide, which potentially coexist in the marine atmosphere, should also be considered in future studies.
METHODS QUANTUM CHEMISTRY CALCULATIONS To fully sample the isomers of each studied IA-DMA cluster, the systematic multi-step conformation search was adopted here to obtain the cluster
structures with minimum energy (Supplementary Methods). The final lowest-lying structure were optimized at _ω_B97XD/6-311++G(3df,3pd) (for C, H, O, N) + aug-cc-pVTZ-PP with ECP28MDF (for I)
level of theory39,40. In addition to IA-DMA clusters, the employed IA-NH3 cluster structures were referred to the previous studies35,41 and reoptimized at the employed level of theory in
this study. The structures with lower Gibbs free energies were retained and the corresponding Cartesian coordinates are summarized in Supplementary Table 5. All structural optimizations with
tight convergence criteria (Integration grid: FineGrid) and frequency calculations were performed by density functional theory (DFT) using Gaussian 09 program42. Furthermore, the
single-point correction was performed by RI-CC2 method43 with aug-cc-pVTZ44 (for C, H, O, N) + aug-cc-pVTZ-PP with ECP28MDF (for I) basis set45 using Turbomole program46, since there is
evidence that ACDC simulations based on RI-CC2 values are in better agreement with the experimental results47,48,49. Herein, in the present work, the Gibbs formation free energy (∆_G_) of
the IA-DMA and IA-NH3 cluster is calculated as Eq. (2): $${\Delta}G = {\Delta}E_{{{{\mathrm{RI}}}} - {{{\mathrm{CC}}}}2} + {\Delta}G_{{{{\mathrm{thermal}}}}}^{\omega
{{{\mathrm{B}}}}97{{{\mathrm{X}}}} - {{{\mathrm{D}}}}}$$ (2) where ∆_G_thermal is the thermal contribution and Δ_E_ is the electronic contribution to Gibbs free energy. To simulate
nucleation at varying temperatures, the ∆_G_ of IA-DMA clusters at _T_ = 218–298 K were calculated by Shermo50 and collected in Supplementary Table 2. In addition, considering the effect of
vapor concentration30, the actual Gibbs free energy was also calculated as Eq. (3): $${\Delta}G\left( {P_1,P_2,...,P_n} \right) = {\Delta}G_{\rm{ref}} - k_{\rm{B}}T\mathop {\sum}\limits_{i =
1}^n {N_i} \ln \left( {\frac{{P_i}}{{P_{\rm{ref}}}}} \right)$$ (3) where Δ_G_ref is calculated at the reference pressure _P_ref (1 atm), _k_B is the Boltzmann constant, _n_ is the number of
components in the cluster, _N__i_ is the number of molecules _i_ within the cluster and _P__i_ is the partial pressure of component _i_. WAVEFUNCTION ANALYSIS Intermolecular interactions
can intrinsically affect the stability of the formed cluster. Hence, to better understand the binding nature, the identified cluster structure was investigated by wavefunction analysis using
Multiwfn 3.851. Specifically, the electrostatic potential (ESP) on the molecular vdW surface was calculated to identify potential interaction sites. The reduced density gradient (RDG)52
analysis was conducted to characterize non-covalent interactions. To further quantify the strength of interactions, electron density _ρ_(_r_), Laplacian electron density \(\nabla\)2_ρ_(_r_),
energy density _H_(_r_) at corresponding bond critical points (BCPs) based on atoms in molecules (AIM) theory53 were also calculated in this study (Supplementary Table 1). ATMOSPHERIC
CLUSTER DYNAMIC SIMULATIONS The Atmospheric Clusters Dynamic Code (ACDC)28 derives the steady-state concentration and cluster formation rates by solving the birth-death equation (Eq. (4) as
below) of the studied clusters. For each ACDC simulation, all possible collision and evaporation processes were considered in this study. $$\begin{array}{l}\frac{dC_i}{dt} =
\frac{1}{2}\mathop {\sum}\limits_{j {< i}} {\beta _{j,( {i - j})}C_jC_{( {i - j})} +} \mathop {\sum}\limits_j {\gamma _{( {i + j}) \to i}C_{i{{{\mathrm{ + }}}}j}}\\ \qquad- \mathop
{\sum}\limits_j {\beta _{i,j}C_i} C_j - \frac{1}{2}\mathop {\sum}\limits_{j < i} {\gamma _{i \to j}C_i} + Q_i - S_i\end{array}$$ (4) where _β__i_,_j_ is the collision rate coefficient
between clusters _i_ and _j_, _C__i_ is the concentration of cluster _i_, _γ__i_,_j_ is the evaporation rate coefficient of a cluster (_i_ + _j_) evaporating into smaller clusters _i_ and
_j_, _Q__i_ is an outside source term of cluster _i_, and _S__i_ is the other possible sink term of cluster _i_. Details for calculating _β__i_,_j_ and _γ__i_,_j_ is presented in
Supplementary Methods. In the ACDC simulations, the growth of clusters out of the simulated system depends on whether the rate at which clusters collide with monomers exceeds their own
evaporation (_βC_/∑_γ_ >1)54. Once the boundary clusters (the smallest ones out of the system) are formed, they tend to keep growing without evaporating into smaller sizes28. Here, the
boundary clusters in the IA-DMA system are set to be (IA)7, (IA)5.(DMA)2 and (IA)6.(DMA)1 clusters (see Supplementary Methods). For the IA-NH3 system, the boundary clusters are set to be
(IA)7 and (IA)6.(NH3)1 clusters with reference to previous study35. Moreover, the effect of different condensation sink coefficients (1.0 × 10−2–1.0 × 10−4 s−1), covering coastal to polar
regions8,14,32, on the results was examined in Supplementary Figs. 4, 5. DATA AVAILABILITY All data were available in the main text or supplementary materials. All other relevant data are
available from the corresponding authors upon reasonable request. CODE AVAILABILITY ACDC code is publicly available at https://github.com/tolenius/ACDC. REFERENCES * Collins, M. et al.
Long-term climate change: projections, commitments and irreversibility. in Climate Change 2013-The Physical Science Basis: Contribution of Working Group I to the Fifth Assessment Report of
the Intergovernmental Panel on Climate Change 1029–1136 (Cambridge University Press, 2013). * Takegawa, N. et al. Enhanced New Particle Formation Above the Marine Boundary Layer Over the
Yellow Sea: Potential Impacts on Cloud Condensation Nuclei. _J. Geophys. Res. Atmos_. 125, (2020). * Kulmala, M. How particles nucleate and grow. _Science_ 302, 1000–1001 (2003). Article
Google Scholar * Kulmala, M. et al. Direct observations of atmospheric aerosol nucleation. _Science_ 339, 943–946 (2013). Article Google Scholar * O’Dowd, C. D. & de Leeuw, G. Marine
aerosol production: a review of the current knowledge. _Philos. Trans. R. Soc. A._ 365, 1753–1774 (2007). Article Google Scholar * Zhang, R., Khalizov, A., Wang, L., Hu, M. & Xu, W.
Nucleation and growth of nanoparticles in the atmosphere. _Chem. Rev._ 112, 1957–2011 (2012). Article Google Scholar * Kerminen, V.-M. et al. Atmospheric new particle formation and growth:
review of field observations. _Environ. Res. Lett._ 13, 103003 (2018). Article Google Scholar * Yu, H. et al. Iodine speciation and size distribution in ambient aerosols at a coastal new
particle formation hotspot in China. _Atmos. Chem. Phys._ 19, 4025–4039 (2019). Article Google Scholar * McFiggans, G. et al. Direct evidence for coastal iodine particles from Laminaria
macroalgae – linkage to emissions of molecular iodine. _Atmos. Chem. Phys._ 4, 701–713 (2004). Article Google Scholar * Saiz-Lopez, A. et al. Atmospheric chemistry of iodine. _Chem. Rev._
112, 1773–1804 (2012). Article Google Scholar * Baccarini, A. et al. Frequent new particle formation over the high Arctic pack ice by enhanced iodine emissions. _Nat. Commun._ 11, 4924
(2020). Article Google Scholar * Sipilä, M. et al. Molecular-scale evidence of aerosol particle formation via sequential addition of HIO3. _Nature_ 537, 532–534 (2016). Article Google
Scholar * Beck, L. J. et al. Differing mechanisms of new particle formation at two arctic sites. _Geophys. Res. Lett._ 48, e2020GL091334 (2021). Article Google Scholar * He, X.-C. et al.
Role of iodine oxoacids in atmospheric aerosol nucleation. _Science_ 371, 589–595 (2021). Article Google Scholar * Almeida, J. et al. Molecular understanding of sulphuric acid-amine
particle nucleation in the atmosphere. _Nature_ 502, 359–363 (2013). Article Google Scholar * Olenius, T. et al. New particle formation from sulfuric acid and amines: comparison of
monomethylamine, dimethylamine, and trimethylamine. _J. Geophys. Res. Atmos._ 122, 7103–7118 (2017). Article Google Scholar * Bork, N., Elm, J., Olenius, T. & Vehkamäki, H. Methane
sulfonic acid-enhanced formation of molecular clusters of sulfuric acid and dimethyl amine. _Atmos. Chem. Phys._ 14, 12023–12030 (2014). Article Google Scholar * Shen, J. et al. Structural
effects of amines in enhancing methanesulfonic acid-driven new particle formation. _Environ. Sci. Technol._ 54, 13498–13508 (2020). Article Google Scholar * Yao, L. et al. Atmospheric new
particle formation from sulfuric acid and amines in a Chinese megacity. _Science_ 361, 278–281 (2018). Article Google Scholar * Youn, J.-S., Crosbie, E., Maudlin, L. C., Wang, Z. &
Sorooshian, A. Dimethylamine as a major alkyl amine species in particles and cloud water: Observations in semi-arid and coastal regions. _Atmos. Environ._ 122, 250–258 (2015). Article
Google Scholar * Facchini, M. C. et al. Important source of marine secondary organic aerosol from biogenic amines. _Environ. Sci. Technol._ 42, 9116–9121 (2008). Article Google Scholar *
Brean, J. et al. Open ocean and coastal new particle formation from sulfuric acid and amines around the Antarctic Peninsula. _Nat. Geosci._ 14, 383–388 (2021). Article Google Scholar * van
Pinxteren, M. et al. Aliphatic amines at the Cape Verde atmospheric observatory: abundance, origins and sea-air fluxes. _Atmos. Environ._ 203, 183–195 (2019). Article Google Scholar *
Grönberg, L., Lövkvist, P. & Jönsson, J. Å. Measurement of aliphatic amines in ambient air and rainwater. _Chemosphere_ 24, 1533–1540 (1992). Article Google Scholar * Van Neste, A.,
Duce, R. A. & Lee, C. Methylamines in the marine atmosphere. _Geophys. Res. Lett._ 14, 711–714 (1987). Article Google Scholar * Gibb, S. W., Mantoura, R. F. C. & Liss, P. S.
Ocean-atmosphere exchange and atmospheric speciation of ammonia and methylamines in the region of the NW Arabian Sea. _Glob. Biogeochem. Cycles_ 13, 161–178 (1999). Article Google Scholar
* Quéléver, L. L. J. et al. Investigation of new particle formation mechanisms and aerosol processes at Marambio Station, Antarctic Peninsula. _Atmos. Chem. Phys._ 22, 8417–8437 (2022).
Article Google Scholar * McGrath, M. J. et al. Atmospheric cluster dynamics code: a flexible method for solution of the birth-death equations. _Atmos. Chem. Phys._ 12, 2345–2355 (2012).
Article Google Scholar * Rozas, I., Alkorta, I. & Elguero, J. Behavior of ylides containing N, O, and C atoms as hydrogen bond acceptors. _J. Am. Chem. Soc._ 122, 11154–11161 (2000).
Article Google Scholar * Vehkamäki, H. _Classical Nucleation Theory in Multicomponent Systems_ (Springers, 2006). * Wentworth, G. R. et al. Ammonia in the summertime Arctic marine boundary
layer: sources, sinks, and implications. _Atmos. Chem. Phys._ 16, 1937–1953 (2016). Article Google Scholar * Dal Maso, M. et al. Condensation and coagulation sinks and formation of
nucleation mode particles in coastal and boreal forest boundary layers. _J. Geophys. Res Atmos_. 107, PAR 2-1–PAR 2-10 (2002). Article Google Scholar * Yu, F. & Luo, G. Modeling of
gaseous methylamines in the global atmosphere: impacts of oxidation and aerosol uptake. _Atmos. Chem. Phys._ 14, 12455–12464 (2014). Article Google Scholar * Norman, M. Distribution of
marine boundary layer ammonia over the Atlantic and Indian Oceans during the Aerosols99 cruise. _J. Geophys. Res._ 110, D16302 (2005). Article Google Scholar * Rong, H. et al. Nucleation
mechanisms of iodic acid in clean and polluted coastal regions. _Chemosphere_ 253, 126743 (2020). Article Google Scholar * NASA POWER. Data access viewer.
https://power.larc.nasa.gov/data-access-viewer/ (2022). * Khanniche, S., Louis, F., Cantrel, L. & Černušák, I. A theoretical study of the microhydration of iodic acid (HOIO2). _Comput.
Theor. Chem._ 1094, 98–107 (2016). Article Google Scholar * Paasonen, P. et al. On the formation of sulphuric acid – amine clusters in varying atmospheric conditions and its influence on
atmospheric new particle formation. _Atmos. Chem. Phys._ 12, 9113–9133 (2012). Article Google Scholar * Francl, M. M. et al. Self‐consistent molecular orbital methods. XXIII. A
polarization‐type basis set for second‐row elements. _J. Chem. Phys._ 77, 3654–3665 (1982). Article Google Scholar * Peterson, K. A., Figgen, D., Goll, E., Stoll, H. & Dolg, M.
Systematically convergent basis sets with relativistic pseudopotentials. II. Small-core pseudopotentials and correlation consistent basis sets for the post- d group 16–18 elements. _J. Chem.
Phys._ 119, 11113–11123 (2003). Article Google Scholar * Xia, D. et al. Formation mechanisms of iodine–ammonia clusters in polluted coastal areas unveiled by thermodynamics and kinetic
simulations. _Environ. Sci. Technol._ 54, 9235–9242 (2020). Article Google Scholar * Frisch, M. J. et al. Gaussian 09 revision A.1. (2009). * Hattig, C. & Weigend, F. CC2 excitation
energy calculations on large molecules using the resolution of the identity approximation. _J. Chem. Phys._ 113, 5154–5161 (2000). Article Google Scholar * Kendall, R. A., Dunning, T. H.
& Harrison, R. J. Electron affinities of the first‐row atoms revisited. Systematic basis sets and wave functions. _J. Chem. Phys._ 96, 6796–6806 (1992). Article Google Scholar *
Pritchard, B. P., Altarawy, D., Didier, B., Gibson, T. D. & Windus, T. L. New basis set exchange: an open, up-to-date resource for the molecular sciences community. _J. Chem. Inf.
Model._ 59, 4814–4820 (2019). Article Google Scholar * Ahlrichs, R., Bär, M., Häser, M., Horn, H. & Kölmel, C. Electronic structure calculations on workstation computers: the program
system turbomole. _Chem. Phys. Lett._ 162, 165–169 (1989). Article Google Scholar * Kürten, A. et al. New particle formation in the sulfuric acid–dimethylamine–water system: reevaluation
of CLOUD chamber measurements and comparison to an aerosol nucleation and growth model. _Atmos. Chem. Phys._ 18, 845–863 (2018). Article Google Scholar * Carlsson, P. T. M. et al. Neutral
sulfuric acid–water clustering rates: bridging the gap between molecular simulation and experiment. _J. Phys. Chem. Lett._ 11, 4239–4244 (2020). Article Google Scholar * Li, H. et al.
Influence of atmospheric conditions on sulfuric acid-dimethylamine-ammonia-based new particle formation. _Chemosphere_ 245, 125554 (2020). Article Google Scholar * Lu, T. & Chen, Q.
Shermo: a general code for calculating molecular thermochemistry properties. _Comput. Theor. Chem._ 1200, 113249 (2021). Article Google Scholar * Lu, T. & Chen, F. Multiwfn: a
multifunctional wavefunction analyzer. _J. Comput. Chem._ 33, 580–592 (2012). Article Google Scholar * Johnson, E. R. et al. Revealing noncovalent interactions. _J. Am. Chem. Soc._ 132,
6498–6506 (2010). Article Google Scholar * Becke, A. _The Quantum Theory of Atoms in Molecules: From Solid State to DNA and Drug Design_ (Wiley, 2007). * Elm, J. et al. Formation of
atmospheric molecular clusters consisting of sulfuric acid and C8H12O6 tricarboxylic acid. _Phys. Chem. Chem. Phys._ 19, 4877–4886 (2017). Article Google Scholar Download references
ACKNOWLEDGEMENTS This work was supported by the National Natural Science Foundation of China [grant number 21976015] and the National Science Fund for Distinguished Young Scholars [grant
number 22225607]. L. Liu thanks the National Natural Science Foundation of China [grant number 42105101]. We acknowledge the National Super Computing Center in Shenzhen for providing the
computational resources and Turbomole program. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Key Laboratory of Cluster Science, Ministry of Education of China, School of Chemistry and
Chemical Engineering, Beijing Institute of Technology, Beijing, 100081, China An Ning, Ling Liu, Shaobing Zhang & Xiuhui Zhang * Atmospheric Sciences Research Center, University at
Albany, Albany, New York, NY, 12203, USA Fangqun Yu * Environment Research Institute, Shandong University, Qingdao, 266237, China Lin Du * State Key Laboratory for Structural Chemistry of
Unstable and Stable Species, CAS Research/Education Center for Excellence in Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190, China Maofa Ge Authors
* An Ning View author publications You can also search for this author inPubMed Google Scholar * Ling Liu View author publications You can also search for this author inPubMed Google Scholar
* Shaobing Zhang View author publications You can also search for this author inPubMed Google Scholar * Fangqun Yu View author publications You can also search for this author inPubMed
Google Scholar * Lin Du View author publications You can also search for this author inPubMed Google Scholar * Maofa Ge View author publications You can also search for this author inPubMed
Google Scholar * Xiuhui Zhang View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS X.Z. contributed to conceiving the idea, analyzing results,
editing, and revision. A.N. contributed to performing DFT and ACDC calculations, analyzing results and interpreting data, and writing the original draft. L.L., S.Z., L.D., and M.G.
contributed to editing and revision. F.Y. contributed to performing GEOS-Chem simulations and editing. CORRESPONDING AUTHOR Correspondence to Xiuhui Zhang. ETHICS DECLARATIONS COMPETING
INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTAL MATERIAL RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International
License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source,
provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons
license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by
statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Ning, A., Liu, L., Zhang, S. _et al._ The critical role of dimethylamine in the
rapid formation of iodic acid particles in marine areas. _npj Clim Atmos Sci_ 5, 92 (2022). https://doi.org/10.1038/s41612-022-00316-9 Download citation * Received: 12 March 2022 * Accepted:
04 November 2022 * Published: 17 November 2022 * DOI: https://doi.org/10.1038/s41612-022-00316-9 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative