Nickel–molybdenum–niobium metallic glass for efficient hydrogen oxidation in hydroxide exchange membrane fuel cells
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

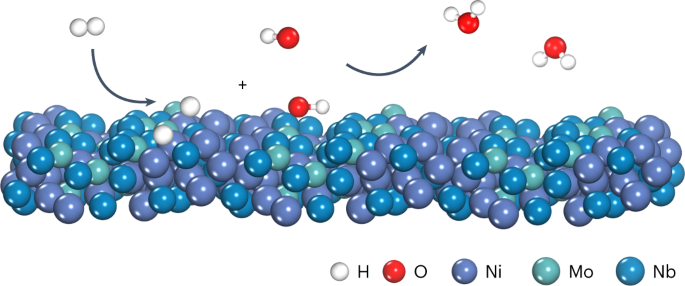
ABSTRACT The cost of fuel cell systems can be largely reduced by developing hydroxide exchange membrane fuel cells (HEMFCs) based on platinum group metal-free (PGM-free) catalysts. However,
the sluggish hydrogen oxidation reaction (HOR) in alkaline electrolytes forces HEMFCs to use higher PGM loadings at the anode than proton exchange membrane fuel cells to sustain the desired
power densities. Here we report nickel–molybdenum–niobium metallic glasses as PGM-free HOR catalysts. The optimal Ni52Mo13Nb35 metallic glass exhibits an intrinsic exchange current density
of 0.35 mA cm−2, outperforming that of a Pt disk catalyst (0.30 mA cm−2). This catalyst also shows remarkable robustness in alkaline electrolyte with a wide stability window up to 0.8 V
versus the reversible hydrogen electrode. When used as the anode, this catalyst enables power densities of 390 mW cm−2 in H2/O2 fuel cells and 253 mW cm−2 in H2/air fuel cells, and shows
negligible performance degradation over 50 h and 30 h, respectively. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution
ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $32.99 / 30 days cancel any time
Learn more Subscribe to this journal Receive 12 digital issues and online access to articles $119.00 per year only $9.92 per issue Learn more Buy this article * Purchase on SpringerLink *
Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional
subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS AN EFFICIENT NICKEL HYDROGEN OXIDATION CATALYST FOR HYDROXIDE EXCHANGE MEMBRANE FUEL CELLS
Article 04 April 2022 TERNARY NICKEL–TUNGSTEN–COPPER ALLOY RIVALS PLATINUM FOR CATALYZING ALKALINE HYDROGEN OXIDATION Article Open access 11 May 2021 BIMETALLIC NICKEL-MOLYBDENUM/TUNGSTEN
NANOALLOYS FOR HIGH-EFFICIENCY HYDROGEN OXIDATION CATALYSIS IN ALKALINE ELECTROLYTES Article Open access 22 September 2020 DATA AVAILABILITY The data that support the findings of this study
are presented in the article and Supplementary Information. Source data are provided with this paper. Any other relevant data are also available from the corresponding authors upon
reasonable request. REFERENCES * Sekine, S. & Kojima, K. Progress and challenges in Toyota’s fuel cell vehicle development. In _16th Asia Pacific Automotive Engineering Conference_
https://doi.org/10.4271/2011-28-0061 (The Automotive Research Association of India, 2011). * Shinji Jomori, K. K., Nonoyama, N. & Manabu Kato, T. Y. Experimental study of the activity
change due to operation history in PEMFC. _Electrochem. Soc._ 58, 1457–1469 (2013). Google Scholar * Chattot, R. et al. Surface distortion as a unifying concept and descriptor in oxygen
reduction reaction electrocatalysis. _Nat. Mater._ 17, 827–833 (2018). Article CAS PubMed PubMed Central Google Scholar * Setzler, B. P., Zhuang, Z., Wittkopf, J. A. & Yan, Y.
Activity targets for nanostructured platinum-group-metal-free catalysts in hydroxide exchange membrane fuel cells. _Nat. Nanotechnol._ 11, 1020–1025 (2016). Article CAS PubMed Google
Scholar * Strmcnik, D. et al. Improving the hydrogen oxidation reaction rate by promotion of hydroxyl adsorption. _Nat. Chem._ 5, 300–306 (2013). Article CAS PubMed Google Scholar *
Sheng, W., Gasteiger, H. A. & Shao-Horn, Y. Hydrogen oxidation and evolution reaction kinetics on platinum: acid vs alkaline electrolytes. _J. Electrochem. Soc._ 157, B1529–B1536 (2010).
Article CAS Google Scholar * Tatus-Portnoy, Z. et al. A low-loading Ru-rich anode catalyst for high-power anion exchange membrane fuel cells. _Chem. Commun._ 56, 5669–5672 (2020).
Article CAS Google Scholar * Rheinländer, P. J., Herranz, J., Durst, J. & Gasteiger, H. A. Kinetics of the hydrogen oxidation/evolution reaction on polycrystalline platinum in
alkaline electrolyte reaction order with respect to hydrogen pressure. _J. Electrochem. Soc._ 161, F1448–F1457 (2014). Article Google Scholar * Zhuang, Z. et al. Nickel supported on
nitrogen-doped carbon nanotubes as hydrogen oxidation reaction catalyst in alkaline electrolyte. _Nat. Commun._ 7, 10141 (2016). Article CAS PubMed PubMed Central Google Scholar *
Davydova, E. S., Mukerjee, S., Jaouen, F. & Dekel, D. R. Electrocatalysts for hydrogen oxidation reaction in alkaline electrolytes. _ACS Catal._ 8, 6665–6690 (2018). 2018. Article CAS
Google Scholar * Dekel, D. R. Review of cell performance in anion exchange membrane fuel cells. _J. Power Sources_ 375, 158–169 (2018). Article CAS Google Scholar * Cherstiouk, O. V. et
al. Electrocatalysis of the hydrogen oxidation reaction on carbon-supported bimetallic NiCu particles prepared by an improved wet chemical synthesis. _J. Electroanal. Chem._ 783, 146–151
(2016). Article CAS Google Scholar * Gao, L. et al. A nickel nanocatalyst within a h-BN shell for enhanced hydrogen oxidation reactions. _Chem. Sci._ 8, 5728–5734 (2017). Article CAS
PubMed PubMed Central Google Scholar * Kiros, Y., Majari, M. & Nissinen, T. A. Effect and characterization of dopants to Raney nickel for hydrogen oxidation. _J. Alloys Compd._ 360,
279–285 (2003). Article CAS Google Scholar * Ni, W. et al. Ni3N as an active hydrogen oxidation reaction catalyst in alkaline medium. _Angew. Chem. Int. Ed._ 58, 7445–7449 (2019). Article
CAS Google Scholar * Yang, Y. et al. Enhanced electrocatalytic hydrogen oxidation on Ni/NiO/C derived from a Ni-based MOF. _Angew. Chem. Int. Ed._ 131, 10754–10759 (2019). Article
Google Scholar * Kabir, S. et al. Platinum group metal-free NiMo hydrogen oxidation catalysts: high performance and durability in alkaline exchange membrane fuel cells. _J. Mater. Chem. A_
5, 24433–24443 (2017). Article CAS Google Scholar * Sheng, W. et al. Non-precious metal electrocatalysts with high activity for hydrogen oxidation reaction in alkaline electrolytes.
_Energy Environ. Sci._ 7, 1719–1724 (2014). Article CAS Google Scholar * Yang, F. et al. Boosting hydrogen oxidation activity of Ni in alkaline media through oxygen-vacancy-rich CeO2/Ni
heterostructures. _Angew. Chem. Int. Ed._ 58, 14179–14183 (2019). Article CAS Google Scholar * Lu, S., Pan, J., Huang, A., Zhuang, L., & Lu, J. Alkaline polymer electrolyte fuel cells
completely free from noble metal catalysts. _Proc. Natl Acad. Sci. USA_ 105, 20611–20614 (2008). Article CAS PubMed Central Google Scholar * Song, F. et al. Interfacing nickel nitride
and nickel boosts both electrocatalytic hydrogen evolution and oxidation reactions. _Nat. Commun._ 9, 4531 (2018). Article PubMed PubMed Central Google Scholar * Duan, Y. et al.
Bimetallic nickel-molybdenum/tungsten nanoalloys for high-efficiency hydrogen oxidation catalysis in alkaline electrolytes. _Nat. Commun._ 11, 4789 (2020). Article CAS PubMed PubMed
Central Google Scholar * Davydova, E. S., Speck, F. D., Paul, M. T. Y., Dekel, D. R. & Cherevko, S. Stability limits of Ni-based hydrogen oxidation electrocatalysts for anion exchange
membrane fuel cells. _ACS Catal._ 9, 6837–6845 (2019). Article CAS Google Scholar * Qin, S. et al. Ternary nickel–tungsten–copper alloy rivals platinum for catalyzing alkaline hydrogen
oxidation. _Nat. Commun._ 12, 2686 (2021). Article CAS PubMed PubMed Central Google Scholar * Oshchepkov, A. G. et al. Nanostructured nickel nanoparticles supported on vulcan carbon as
a highly active catalyst for the hydrogen oxidation reaction in alkaline media. _J. Power Sources_ 402, 447–452 (2018). Article CAS Google Scholar * Davydova, E., Zaffran, J., Dhaka, K.,
Toroker, M. & Dekel, D. Hydrogen oxidation on Ni-based electrocatalysts: the effect of metal doping. _Catalysts_ 8, 454 (2018). Article Google Scholar * Winsel, E. W. & Ja, A. W.
The DSK system of fuel cell electrodes. _J. Electrochem. Soc._ 108, 1073–1079 (1961). Article Google Scholar * Roy, A. et al. Nickel–copper supported on a carbon black hydrogen oxidation
catalyst integrated into an anion-exchange membrane fuel cell. _Sustain. Energy Fuels_ 2, 2268–2275 (2018). Article CAS Google Scholar * Klement, W., Willens, R. H. & Duwez, P.
Non-crystalline structure in solidified gold-silicon alloys. _Nature_ 187, 869–870 (1960). Article CAS Google Scholar * Greer, A. L. Metallic glasses…on the threshold. _Mater. Today_ 12,
14–22 (2009). Article CAS Google Scholar * Escudero-Escribano, M. et al. Tuning the activity of Pt alloy electrocatalysts by means of the lanthanide contraction. _Science_ 352, 73–76
(2016). Article CAS PubMed Google Scholar * Zhang, X. et al. Lithiation-induced amorphization of Pd3P2S8 for highly efficient hydrogen evolution. _Nat. Catal._ 1, 460–468 (2018). Article
CAS Google Scholar * Zhang, B. et al. Homogeneously dispersed multimetal oxygen-evolving catalysts. _Science_ 352, 333–337 (2016). Article CAS PubMed Google Scholar * Carmo, M. et
al. Bulk Metallic glass nanowire architecture for electrochemical applications. _ACS Nano_ 5, 2979–2298 (2011). Article CAS PubMed Google Scholar * Li, J. et al. Combinatorial screening
of Pd-based quaternary electrocatalysts for oxygen reduction reaction in alkaline media. _J. Mater. Chem. A_ 5, 67–72 (2017). Article CAS Google Scholar * Sekol, R. C. et al. Bulk
metallic glass micro fuel cell. _Small_ 9, 2081–2085 (2013) 2026. Article CAS PubMed Google Scholar * Schilter, D., Fuller, A. L. & Gray, D. L. Nickel-molybdenum and nickel-tungsten
dithiolates: hybrid models for hydrogenases and hydrodesulfurization. _Eur. J. Inorg. Chem._ 2015, 4638–4642 (2015). Article CAS Google Scholar * Inoue, A. & Ta, A. Classification of
bulk metallic glasses by atomic size difference, heat of mixing and period of constituent elements and its application to characterization of the main alloying element. _Mater. Trans._ 46,
2817–2829 (2005). Article Google Scholar * Wang, Y., Brezesinski, T., Antonietti, M. & Smarsly, B. Ordered mesoporous Sb-, Nb-, and Ta-doped SnO2 thin films with adjustable doping
levels and high electrical conductivity. _ACS Nano_ 3, 1373–1378 (2009). Article CAS PubMed Google Scholar * Wei, X. et al. In situ visualizing atomic structural evolution during
crystallization in ternary Zr–Cu–Al bulk metallic glasses. _Intermetallics_ 105, 173–178 (2019). Article CAS Google Scholar * Fernandez Garrillo, P. A., Grevin, B., Chevalier, N. &
Borowik, L. Calibrated work function mapping by Kelvin probe force microscopy. _Rev. Sci. Instrum._ 89, 043702 (2018). Article PubMed Google Scholar * Ashby, M. & Greer, A. Metallic
glasses as structural materials. _Scr. Mater._ 54, 321–326 (2006). Article CAS Google Scholar * Johnson, W. L. Bulk glass-forming metallic alloys: science and technology. _MRS Bull._ 24,
42–56 (1999). Article CAS Google Scholar * Schroers, J. Bulk metallic glasses. _Phys. Today_ 66, 32–37 (2013). Article CAS Google Scholar * Lan, S. et al. A medium-range structure
motif linking amorphous and crystalline states. _Nat. Mater._ 20, 1347–1352 (2021). Article CAS PubMed Google Scholar * Lan, S. et al. Hidden amorphous phase and reentrant supercooled
liquid in Pd-Ni-P metallic glasses. _Nat. Commun._ 8, 14679 (2017). Article CAS PubMed PubMed Central Google Scholar * Kube, S. A. et al. Compositional dependence of the fragility in
metallic glass forming liquids. _Nat. Commun._ 13, 3708 (2022). Article CAS PubMed PubMed Central Google Scholar * Zhukov, A. et al. Trends in optimization of giant magnetoimpedance
effect in amorphous and nanocrystalline materials. _J. Alloys Compd._ 727, 887–901 (2017). Article CAS Google Scholar * Inoue, A. & Nishiyama, N. New bulk metallic glasses for
applications as magnetic-sensing, chemical, and structural materials. _MRS Bull._ 32, 651–658 (2007). Article CAS Google Scholar * Glasscott, M. W. et al. Electrosynthesis of high-entropy
metallic glass nanoparticles for designer, multi-functional electrocatalysis. _Nat. Commun._ 10, 2650 (2019). Article PubMed PubMed Central Google Scholar * Tan, Y. et al.
Noble‐metal‐free metallic glass as a highly active and stable bifunctional electrocatalyst for water splitting. _Adv. Mater. Interfaces_ 4, 1601086 (2017). Article Google Scholar * Bard,
A. J. & Faulkner, L. R. _Electrochemical Methods: Fundamentals and Applications_ (Wiley, 2001). * Sun, Y., Dai, Y., Liu, Y. & Chen, S. A rotating disk electrode study of the particle
size effects of Pt for the hydrogen oxidation reaction. _Phys. Chem. Chem. Phys._ 14, 2278–2285 (2012). Article CAS PubMed Google Scholar * Cong, Y., Yi, B. & Song, Y. Hydrogen
oxidation reaction in alkaline media: from mechanism to recent electrocatalysts. _Nano Energy_ 44, 288–303 (2018). Article CAS Google Scholar * Camara, G. A., Ticianelli, E. A., Mukerjee,
S., Lee, J. & McBreen, J. The CO poisoning mechanism of the hydrogen oxidation reaction in proton exchange membrane fuel cells. _J. Electrochem. Soc._ 149, A748–A753 (2002). Article
CAS Google Scholar * Wu, G. et al. A general synthesis approach for amorphous noble metal nanosheets. _Nat. Commun._ 10, 4855 (2019). Article PubMed PubMed Central Google Scholar *
Sheng, H. W., Luo, W. K., Alamgir, F. M., Bai, J. M. & Ma, E. Atomic packing and short-to-medium-range order in metallic glasses. _Nature_ 439, 419–425 (2006). Article CAS PubMed
Google Scholar * Gross, O. et al. Signatures of structural differences in Pt–P- and Pd–P-based bulk glass-forming liquids. _Commun. Phys._ 2, 83 (2019). Article Google Scholar * Ding, J.,
Ma, E., Asta, M. & Ritchie, R. O. Second-nearest-neighbor correlations from connection of atomic packing motifs in metallic glasses and liquids. _Sci. Rep._ 5, 17429 (2015). Article
CAS PubMed PubMed Central Google Scholar * Miracle, D. B. A structural model for metallic glasses. _Nat. Mater._ 3, 697–702 (2004). Article CAS PubMed Google Scholar * Ma, D.,
Stoica, A. D. & Wang, X. L. Power-law scaling and fractal nature of medium-range order in metallic glasses. _Nat. Mater._ 8, 30–34 (2008). Article PubMed Google Scholar * Giles, S. A.
et al. Recent advances in understanding the pH dependence of the hydrogen oxidation and evolution reactions. _J. Catal._ 367, 328–331 (2018). Article CAS Google Scholar * Zheng, J.,
Nash, J., Xu, B. & Yan, Y. Towards establishing apparent hydrogen binding energy as the descriptor for hydrogen oxidation/evolution reactions. _J. Electrochem. Soc._ 165, H27–H29 (2018).
Article CAS Google Scholar * Cheng, T., Wang, L., Merinov, B. V. & Goddard, W. A. III Explanation of dramatic pH-dependence of hydrogen binding on noble metal electrode: greatly
weakened water adsorption at high pH. _J. Am. Chem. Soc._ 140, 7787–7790 (2018). Article CAS PubMed Google Scholar * Yang, X., Nash, J., Oliveira, N., Yan, Y. & Xu, B. Understanding
the pH dependence of underpotential deposited hydrogen on platinum. _Angew. Chem. Int. Ed._ 58, 17718–17723 (2019). Article CAS Google Scholar * Ledezma-Yanez, I. et al. Interfacial water
reorganization as a pH-dependent descriptor of the hydrogen evolution rate on platinum electrodes. _Nat. Energy_ 2, 17031 (2017). Article CAS Google Scholar * Stevens, M. B. et al.
Ternary Ni-Co-Fe oxyhydroxide oxygen evolution catalysts: intrinsic activity trends, electrical conductivity, and electronic band structure. _Nano Res._ 12, 2288–2295 (2019). Article CAS
Google Scholar * Zaffran, J., Nagli, M., Shehadeh, M. & Toroker, M. C. Efficient cationic agents for exfoliating two-dimensional nickel oxide sheets. _Theor. Chem. Acc._ 137, 3 (2018).
Article Google Scholar * Zaffran, J. & Toroker, M. C. Understanding the oxygen evolution reaction on a two-dimensional NiO2 catalyst. _ChemElectroChem_ 4, 2764–2770 (2017). Article
CAS Google Scholar * Zaffran, J. et al. Influence of electrolyte cations on Ni(Fe)OOH catalyzed oxygen evolution reaction. _Chem. Mater._ 29, 4761–4767 (2017). Article CAS Google Scholar
* Zaffran, J. & Caspary Toroker, M. Benchmarking density functional theory based methods to model NiOOH material properties: Hubbard and van der Waals corrections vs hybrid
functionals. _J. Chem. Theory Comput._ 12, 3807–3812 (2016). Article CAS PubMed Google Scholar * Fidelsky, V., Butera, V., Zaffran, J. & Toroker, M. C. Three fundamental questions on
one of our best water oxidation catalysts: a critical perspective. _Theor. Chem. Acc_. 135, (2016). * Yayapao, O., Phuruangrat, A., Thongtem, T. & Thongtem, S. Synthesis,
characterization and electrochemical properties of α-MoO3 nanobelts for Li-ion batteries. _Russ. J. Phys. Chem. A_ 90, 1224–1230 (2016). Article CAS Google Scholar * Duan, Y. et al.
Scaled-up synthesis of amorphous NiFeMo oxides and their rapid surface reconstruction for superior oxygen evolution catalysis. _Angew. Chem. Int. Ed._ 58, 15772–15777 (2019). Article CAS
Google Scholar * Suryanarayana, C. & Inoue, A. _Bulk Metallic Glasses_ (CRC Press, 2017). * Yoon, Y., Yan, B. & Surendranath, Y. Suppressing ion transfer enables versatile
measurements of electrochemical surface area for intrinsic activity comparisons. _J. Am. Chem. Soc._ 140, 2397–2400 (2018). Article CAS PubMed Google Scholar * Egami, T. & Billinge,
S. _Underneath the Bragg Peaks: Structural Analysis of Complex Materials_ (Pergamon Press, 2003). * Dimitrov, D. A., Röde, H. & Bishop, A. R. Peak positions and shapes in neutron pair
correlation functions from powders of highly anisotropic crystals. _Phys. Rev. B_ 64, 014303 (2001). Article Google Scholar * Kresse, G. & Hafner, J. Ab initio molecular-dynamics
simulation of the liquid-metal–amorphous-semiconductor transition in germanium. _Phys. Rev. B_ 49, 14251–14269 (1994). Article CAS Google Scholar * Kresse, G. & Hafner, J. Ab initio
molecular dynamics for liquid metals. _Phys. Rev. B_ 47, 558–561 (1993). Article CAS Google Scholar * Blochl, P. E. Projector augmented-wave method. _Phys. Rev. B_ 50, 17953–17979 (1994).
Article CAS Google Scholar * Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. _Phys. Rev. Lett._ 77, 3865–3868 (1996). Article CAS PubMed
Google Scholar * Nosé, S. A unified formulation of the constant temperature molecular dynamics methods. _J. Chem. Phys._ 81, 511–519 (1984). Article Google Scholar * Grimme, S., Antony,
J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H–Pu. _J. Chem. Phys._ 132, 154104
(2010). Article PubMed Google Scholar * Grimme, S., Ehrlich, S. & Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. _J. Comput. Chem._ 32,
1456–1465 (2011). Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS We thank S. L. Chen at Wuhan University for many helpful discussions. This work was supported by
the National Basic Research Program of China (grant no. 2018YFA0702001), the National Natural Science Foundation of China (grant nos. 22225901, 22175162, 21975237, 21521001, 21431006,
21225315, 21321002, 91645202 and 51871120), the Chinese Academy of Sciences (grant nos. KGZD-EW-T05 and XDA090301001), the Strategic Priority Research Program of the Chinese Academy of
Sciences (grant no. XDA21000000), the Anhui Provincial Research and Development Program (grant no. 202004a05020073), the USTC Research Funds of the Double First-Class Initiative (grant no.
YD2340002007), the Fundamental Research Funds for the Central Universities (grant nos. WK9990000101, 30919011107 and 30919011404), the Natural Science Foundation of Jiangsu Province (grant
no. BK20200019), the National Key R&D Program of China (grant no. 2021YFB3802800) and Guangdong–Hong Kong–Macao Joint Laboratory for Neutron Scattering Science and Technology. This
research used the resources of the Advanced Photon Source, a US Department of Energy (DOE), Office of Science User Facility operated for the DOE Office of Science by Argonne National
Laboratory under contract no. DE-AC02-06CH11357. AUTHOR INFORMATION Author notes * These authors contributed equally: Fei-Yue Gao, Si-Nan Liu, Jia-Cheng Ge, Xiao-Long Zhang, Li Zhu. AUTHORS
AND AFFILIATIONS * Division of Nanomaterials and Chemistry, Hefei National Laboratory for Physical Sciences at the Microscale, University of Science and Technology of China, Hefei, China
Fei-Yue Gao, Xiao-Long Zhang, Ya-Rong Zheng, Yu Duan, Shuai Qin, Xingxing Yu, Rui-Cheng Bao, Peng-Peng Yang, Zhuang-Zhuang Niu, Min-Rui Gao & Shu-Hong Yu * Herbert Gleiter Institute of
Nanoscience, School of Materials Science and Engineering, Nanjing University of Science and Technology, Nanjing, China Si-Nan Liu, Jia-Cheng Ge, Zhi-Gang Ding, Wei Liu & Si Lan *
Department of Physics, City University of Hong Kong, Hong Kong, China Li Zhu & Weixia Dong * Department of Chemical and Biomolecular Engineering and Center for Catalytic Science and
Technology, University of Delaware, Newark, DE, USA Yushan Yan Authors * Fei-Yue Gao View author publications You can also search for this author inPubMed Google Scholar * Si-Nan Liu View
author publications You can also search for this author inPubMed Google Scholar * Jia-Cheng Ge View author publications You can also search for this author inPubMed Google Scholar *
Xiao-Long Zhang View author publications You can also search for this author inPubMed Google Scholar * Li Zhu View author publications You can also search for this author inPubMed Google
Scholar * Ya-Rong Zheng View author publications You can also search for this author inPubMed Google Scholar * Yu Duan View author publications You can also search for this author inPubMed
Google Scholar * Shuai Qin View author publications You can also search for this author inPubMed Google Scholar * Weixia Dong View author publications You can also search for this author
inPubMed Google Scholar * Xingxing Yu View author publications You can also search for this author inPubMed Google Scholar * Rui-Cheng Bao View author publications You can also search for
this author inPubMed Google Scholar * Peng-Peng Yang View author publications You can also search for this author inPubMed Google Scholar * Zhuang-Zhuang Niu View author publications You can
also search for this author inPubMed Google Scholar * Zhi-Gang Ding View author publications You can also search for this author inPubMed Google Scholar * Wei Liu View author publications
You can also search for this author inPubMed Google Scholar * Si Lan View author publications You can also search for this author inPubMed Google Scholar * Min-Rui Gao View author
publications You can also search for this author inPubMed Google Scholar * Yushan Yan View author publications You can also search for this author inPubMed Google Scholar * Shu-Hong Yu View
author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS M.-R.G. and S.L. conceived the idea and directed the project. S.-H.Y. and Y.Y. advised on the
research and contributed to the scientific interpretation. F.-Y.G., S.-N.L. and J.-C.G. fabricated the metallic glasses and collected and analysed the data. X.-L.Z., L.Z., Z.-G.D. and W.L.
performed the DFT calculations. Y.-R.Z. and Y.D. carried out the XRD measurements. W.D. performed the DMA measurements. R.-C.B. and X.Y. assisted with the electrochemical measurements. S.Q.,
Z.-Z.N. and P.-P.Y. assisted with the structure analyses. F.-Y.G., S.-N.L., J.-C.G., S.L. and M.-R.G. wrote and edited the manuscript. All authors discussed the results and commented on the
manuscript. CORRESPONDING AUTHORS Correspondence to Si Lan or Min-Rui Gao. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. PEER REVIEW PEER REVIEW
INFORMATION _Nature Catalysis_ thanks Hamish Miller, Jan Schroers and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. ADDITIONAL INFORMATION
PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION
Supplementary Discussion, Figs. 1–51 and Tables 1–5. SUPPLEMENTARY DATA Atomic coordinates of the simulated Ni52Mo13Nb35 models. SOURCE DATA SOURCE DATA FIG. 4 The data used to plot the
figures. SOURCE DATA FIG. 5 The data used to plot the figures. RIGHTS AND PERMISSIONS Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with
the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable
law. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Gao, FY., Liu, SN., Ge, JC. _et al._ Nickel–molybdenum–niobium metallic glass for efficient hydrogen oxidation in hydroxide
exchange membrane fuel cells. _Nat Catal_ 5, 993–1005 (2022). https://doi.org/10.1038/s41929-022-00862-8 Download citation * Received: 23 April 2022 * Accepted: 16 September 2022 *
Published: 27 October 2022 * Issue Date: November 2022 * DOI: https://doi.org/10.1038/s41929-022-00862-8 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative