Temporally-regulated quick activation and inactivation of ras is important for olfactory behaviour
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Responses to environmental stimuli are mediated by the activation and inactivation of various signalling proteins. However, the temporal dynamics of these events in living animals
are not well understood. Here we show real-time imaging of the activity of the key regulator of the MAP kinase pathway, Ras, in living _Caenorhabditis elegans_ and that Ras is transiently
activated within a few seconds in olfactory neurons in response to increase in the concentration of odorants. This fast activation of Ras is dependent on the olfactory signalling pathway and
Ras guanyl nucleotide-releasing protein (RasGRP). A negative feedback loop then quickly leads to Ras inactivation despite the continued presence of the odorant. Phenotypes of Ras mutants
suggest this rapid activation and inactivation of Ras is important for regulation of interneuron activities and olfactory behaviours. Our results reveal novel kinetics and biological
implication of transient activation of Ras in olfactory systems. SIMILAR CONTENT BEING VIEWED BY OTHERS MECHANISM OF SENSORY PERCEPTION UNVEILED BY SIMULTANEOUS MEASUREMENT OF MEMBRANE
VOLTAGE AND INTRACELLULAR CALCIUM Article Open access 16 September 2024 HOMEOSTATIC SYNAPTIC PLASTICITY RESCUES NEURAL CODING RELIABILITY Article Open access 24 May 2023 NMDAR-MEDIATED
MODULATION OF GAP JUNCTION CIRCUIT REGULATES OLFACTORY LEARNING IN _C. ELEGANS_ Article Open access 10 July 2020 INTRODUCTION Living organisms employ various signal transduction pathways to
react and adapt to environmental change. These signalling pathways are activated or inactivated in response to extracellular or intracellular stimuli and trigger cellular reactions including
gene expression, cell survival, apoptosis and many biological responses1. Signalling proteins play major roles in such events and constantly alter their status in response to continuous
environmental stimuli. However, temporal dynamics of the signalling proteins in living organisms is largely unknown, even though observation of signalling proteins' activity _in vivo_
is essential for understanding functions and regulatory mechanisms of signal transduction pathways. Ras-MAP kinase pathway is one of the key signal transduction cascades and regulates cell
growth, proliferation, differentiation, neuronal plasticity and others2,3,4. Ras belongs to the small G-protein family and functions as a molecular switch, assuming either the GDP-bound
inactive form or the GTP-bound active form. Mutations of Ras signalling cause many syndromes and diseases, including cancer5. In most cases, Ras is activated in the order of minutes by a
guanine nucleotide exchange factor (GEF)6,7,8 and then activates the MAPK pathway to regulate gene expression. The Ras-MAPK pathway is conserved also in _Caenorhabditis elegans_,
contributing to vulval induction and the other multiple developmental events9. Recently, it has been reported that the Ras-MAPK pathway also plays important roles in the olfactory system of
_C. elegans_10,11. The pathway regulates olfactory sensation in olfactory neurons and olfactory plasticity in interneurons10,11. In olfactory neurons of _C. elegans_, odorants are sensed by
olfactory receptors and these odorant signals are transmitted by G-protein α, guanylate cyclase and cGMP-gated channels12,13,14,15,16. Our previous immunohistochemical study revealed that
MAPK is activated downstream of this olfactory signalling pathway within 10 seconds after an odorant stimulus in olfactory neurons11. This result suggests the possibility that Ras is also
quickly activated in the order of seconds in olfactory neurons, which is distinct from the previously reported kinetics of the Ras activation by growth factors. However, there are as yet
poor understandings about dynamics, regulatory mechanisms and biological implication of the Ras activity in the olfactory system, because the activity of Ras has not yet been observed in
living animals. In this paper, we performed real-time _in vivo_ imaging of the activity of Ras protein and showed quick activation of Ras in response to increase in the concentration of
odorants in olfactory neurons in _C. elegans_. Our study revealed that a negative feedback mechanism downstream of the MAPK pathway leads to quick inactivation of Ras, even when the odorant
stimulation is continued. This activation and inactivation of Ras occurs within a few seconds, which is an unprecedented fast dynamics of Ras activity. Furthermore, measurements of neural
activity by calcium imaging show that Ras signalling in olfactory neurons modulates activity of interneurons which receive synaptic inputs from the olfactory sensory neurons. Finally,
behavioural analyses demonstrate that activation and inactivation of Ras is important for klinotaxis, which is one of the tactics of olfactory behaviour. Our results reveal novel kinetics of
Ras activity and biological significance of the transient activation of Ras in the olfactory system. RESULTS _C. elegans_ senses various odorants and shows attractive or aversive
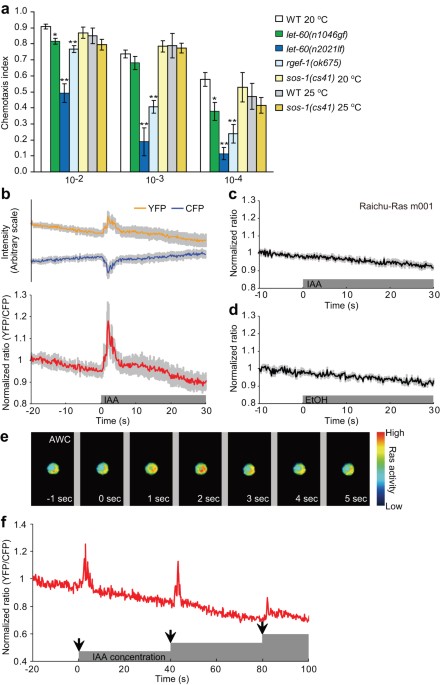
chemotactic behaviour17. As previously reported11, mutations in genes involved in the Ras–MAP kinase (MAPK) pathway cause defects in chemotaxis to attractive odorants. For example, mutant
animals show decreased chemotaxis to isoamyl alcohol, which is sensed by AWC olfactory neurons17,18 (Fig. 1a). MAPK is activated within 10 sec by odorant stimuli in the AWC neurons and the
activity of the Ras-MAPK pathway in AWC is important for behavioural response to the odour11. Constitutive activation of LET-60 Ras in the _let-60(n1046gf)_ mutant, which carries the G13E
mutation19, also reduces olfactory responses11 (Fig. 1a). This suggests that both the activation and inactivation of Ras are important for olfaction; however, the relationship between the
dynamics of Ras activation/inactivation and olfactory behaviours remains unclear. To assess the temporal profile of Ras activation, we expressed Raichu-Ras—a Förster resonance energy
transfer-based biosensor consisting of yellow fluorescent protein (YFP) and cyan fluorescent protein (CFP)20 (Supplementary Fig. S1)—in AWC neurons using the _odr-1_ promoter21, which
allowed us to perform _in vivo_ imaging of Ras activity. Raichu-Ras fluorescence was detected in regions near the plasma membrane of AWC dendrites, axons and cell bodies (Supplementary Fig.
S2a). The transgenic strain did not show any defects in chemotaxis to isoamyl alcohol, suggesting that the AWC olfactory neurons expressing Raichu-Ras functioned normally (Supplementary Fig.
S2b). To image Raichu-Ras signals, transgenic animals were glued on agarose pads, placed into a perfusion chamber and covered with buffer. Then diluted isoamyl alcohol was added to the
buffer (see Methods). Imaging clearly showed an increase in the YFP/CFP emission ratio near the AWC membranes within a few seconds after addition of isoamyl alcohol (Fig. 1b, e,
Supplementary movie S1). After reaching the peak, the ratio decreased to the basal value within 2−3 sec even if the odorant was still present (Fig. 1b, e). These transient changes in the
ratio were reproducibly observed with similar kinetics (Fig. 1b, Supplementary Fig. S3). The changes in YFP and CFP fluorescence intensities opposed each other (Fig. 1b) and ratio changes
were not observed when mutant types of Raichu-Ras (m001, m102, m103) were used (Fig. 1c, Supplementary Fig. S4a, b, d): m001 lacks Raf RBD; m102 contains Y40C mutation in Ras leading to
inhibit the interaction of Ras with Raf; m103 contains S17N mutation reducing the affinity of guanine nucleotides20, which are good indications that the changes in the ratio reflected Ras
activity, rather than being an artefact (Fig. 1b). In contrast, no ratio changes were detected following application of only the solvent, ethanol or pyrazine, which is sensed by AWA
olfactory neurons (Fig. 1d, Supplementary Fig. S4c, d). Ras activation was repeatedly observed after two or three stimulations with stepwise increases of odorant concentration (Fig. 1f). On
the contrary, sham stimuli that did not affect final concentration of the odour failed to activate Ras (Supplementary Fig. S5). These results demonstrate that Ras is transiently activated
owing to an increase in the odorant concentration, implying that the Ras activation/inactivation may be temporally regulated. In the AWC neurons, odorants are thought to be sensed by
seven-transmembrane G protein-coupled receptors and lead to either opening or closure of cyclic nucleotide-gated channel via cyclic GMP second messenger2. Our previous report suggested that
Ras is activated downstream of the olfactory sensory signalling11. To verify that Ras activation depends on the pathway _in vivo_, we monitored Ras activity using Raichu-Ras in
_odr-3(n2150)_ and _tax-2(ks10)_ mutants. _odr-3_ and _tax-2_ encode components of olfactory signalling in AWC neurons: a G protein alpha subunit and a cyclic GMP-gated channel beta subunit,
respectively12,16. In the _odr-3_ and _tax-2_ mutants, Ras activation was not detected after application of isoamyl alcohol (Fig. 2a, b, f). These results suggest that Ras is activated by
odorant stimuli downstream of the olfactory sensory signalling in the AWC neurons. It is widely known that activation of Ras is directly mediated by a Ras-guanine nucleotide exchange factor
(Ras-GEF), which catalyzes the replacement of Ras-bound GDP by GTP6. _C. elegans_ has multiple putative Ras-GEF genes. Among them, SOS-1 has been reported to function as a Ras-GEF during
vulval induction and other processes9 (Supplementary Fig. S6). SOS-1 was required for olfactory plasticity (Supplementary Fig. S7), a process previously reported to be regulated by the
Ras–MAPK pathway10. _sos-1(cs41)_ mutants, however, showed normal chemotaxis to isoamyl alcohol (Fig. 1a). Recently, it has been reported that RGEF-1, an ortholog of mammalian RasGRP, is
required for MAPK activation in olfactory neurons in response to odorant stimuli22. _rgef-1(ok675)_ mutants exhibited significant defects in chemotaxis to isoamyl alcohol (Fig. 1a) and
_rgef-1_ expression in the AWC neurons rescued the defects (Supplementary Fig. S8), as shown in the previous report22, confirming that RGEF-1 functions in AWC olfactory neurons. In the
_rgef-1(ok675)_ mutants, Ras activation was not observed in the AWC neurons (Fig. 2c, f), providing direct evidence that RGEF-1 functions as a Ras-GEF during olfactory reception _in vivo_.
The _rgef-1_ mutants showed normal olfactory plasticity (Supplementary Fig. S7), suggesting that different Ras-GEFs mediate olfactory reception and plasticity in the _C. elegans_ olfactory
system (Supplementary Fig. S6b). The previous report suggested that a diacylglycerol (DAG) signal transports RGEF-1 to membrane regions and activates RGEF-122. EGL-8 (PLCβ) and EGL-30 (Gαq)
are known to be involved in DAG generation and MAPK activation after odour stimuli was significantly reduced in the _egl-8(n488null)_ and _egl-30(ad806lf)_ mutants22. To investigate whether
DAG signals are also important for quick activation of Ras, we monitored Ras activity in the _egl-8(n488)_ and _egl-30(ad806)_ mutants. In these mutants, Ras activation was greatly reduced,
but we noticed that weak but significant activation was reproducibly observed (Fig. 2d-f). These results are qualitatively distinct from that of the _rgef-1_ mutants, in which no consistent
Ras activation was observed (Fig. 2c, f). Therefore both the DAG signal and another signal downstream of odour reception are important for regulation of quick activation of Ras via RGEF-1.
As mentioned above, _let-60(n1046gf)_ mutants showed reduced olfactory behaviour11 (Fig. 1a), indicating that regulation of Ras inactivation is important. Ras activity quickly returned to
the basal level within 2−3 sec after the peak Ras activation, even though the calcium level continued to decrease (Fig. 1b, Supplementary Fig. S3, S9). Therefore, such quick inactivation of
Ras suggests the presence of negative feedback mechanisms. Our previous study demonstrated that MAPK is activated by isoamyl alcohol stimulus in the AWC neurons downstream of Ras11. Thus, to
investigate whether negative feedback mechanisms downstream of Ras modulate Ras inactivation, we analyzed Ras activity in the mutants of _mpk-1_, which encodes MAPK in _C. elegans_23. In
the _mpk-1(ga117null)_ mutants, Ras was normally activated within a few seconds after odour stimulation, but activation longer than that of wild-type animals was observed (Fig. 3a). Indeed,
the average time to peak Ras activation was similar in _mpk-1_ mutants and wild-type animals, whereas the average duration of activation was longer in _mpk-1_ mutants (Fig. 3b). These
results suggest that quick inactivation of Ras is regulated by a negative feedback loop downstream of MAPK. Our previous report suggested that MAPK is activated by odour stimuli in AIY
interneurons and probably regulates olfactory plasticity10, indicating a possibility that functions of MAPK in AIY may affect Ras activity in AWC, because interneuron-mediated feedback
regulation of AWC has been reported previously24. To elucidate whether the quick inactivation of Ras in AWC is controlled by functions of MAPK in AWC sensory neurons or those in AIY
interneurons, we analyzed effects of knockdown of _mpk-1_ functions in AWC or AIY on the Ras activity in AWC. As a result, knockdown of _mpk-1_ functions in AWC of wild type caused
significantly longer duration of the Ras activation, whereas AIY-specific knockdown of _mpk-1_ functions did not affect the Ras inactivation (Fig. 3b, Supplementary Fig. S10). These results
suggest that the inactivation kinetics of Ras is controlled by the MAPK functions in AWC. To clarify the role of MPK-1, we tested chemotaxis of _mpk-1_ mutants. The _mpk-1(ga117null)_
mutants showed severe defects in chemotaxis to isoamyl alcohol (Fig. 3c). The defect of the _mpk-1_ mutants was severer than that of _let-60(lf)_ mutants, probably because _mpk-1(ga117)_ is
a null mutant. The chemotaxis defect of the _mpk-1_ mutants was fully rescued by expression of _mpk-1_ in the AWC neurons but not in AIY interneurons (Fig. 3d). Knockdown of _mpk-1_
functions in AWC caused a severe chemotaxis defect similar to the _mpk-1_ mutants (Fig. 3e). These results suggest that MPK-1 regulates olfactory transduction in the AWC olfactory neurons,
possibly by sending olfactory signals to downstream factors and by sending negative feedback signal to Ras. In conclusion, the Ras activation is regulated by RGEF-1 downstream of the
olfactory sensory signalling and the DAG signal and the quick Ras inactivation is controlled by a negative feedback loop involving MPK-1 in the AWC neurons (Fig. 3f). To assess behaviours
that are regulated by activation and inactivation of Ras, we next monitored chemotaxis behaviours of _let-60_ mutants in detail. _C. elegans_ swimming toward an attractive odorant is
attained of two types of elementary behaviours: klinotaxis and klinokinesis25,26 (Supplementary Fig. S11a, b). We quantified klinotaxis and klinokinesis using the weathervane index and
pirouette index, respectively (see Methods). In our assay, these behaviours were only observed near the odorant (within 30 mm of the odorant) (Fig. 4a, b). As for klinotaxis, both
_let-60(n1046gf)_ and _let-60(n2021lf)_ mutants showed severe defects (Fig. 4a), indicating that proper regulation of Ras activity is essential for regulation of this behavioural mechanism.
The _mpk-1(ga117null)_ mutants also exhibited obvious defects in klinotaxis behaviour (Supplementary Fig. S12). On the other hand, klinokinesis was partially impaired in the _let-60(gf)_ and
_let-60(lf)_ mutants (Fig. 4b). Of note, these defects may reflect, at least in part, an abnormal basal pirouette frequency under odour-free conditions (Fig. 4c). Therefore, though Ras
signalling may also be involved in klinokinesis, these observations indicate that Ras activity clearly regulates klinotaxis behaviour, in which Ras activation in response to increased
odorant concentrations upon head swing may contribute to the curving behaviour toward the odorant. Our results revealed that impaired Ras activation and inactivation cause abnormal
behavioural phenotypes. Next, we sought to determine how Ras signalling affects the activity of the neural circuit that regulates olfactory behaviours. We used calcium imaging to monitor the
activities of AIB interneurons, which receive synaptic inputs from AWC neurons and regulate olfactory behaviours18,25. In AIB neurons, Ca2+ concentrations decreased in response to odour
stimuli (Fig. 5a, g, k), as previously reported18. In the _let-60(gf)_ mutants, the AIB response was not observed (Fig. 5b, k), revealing that constitutive Ras activation disturbed the
responses of downstream interneurons. On the other hand, in the _let-60(lf)_ mutants, unstable calcium concentrations were observed in AIB neurons in the presence of odorant stimuli (Fig.
5c, h). To quantitatively analyze the oscillatory activity of AIB interneurons in the _let-60(lf)_ mutants, we used a Fourier transform which is well suited for revealing oscillatory
responses. Spectral analysis showed significant increase in the band power in a low-frequency domain (0.03–1.0 Hz) in the _let-60(lf)_ mutants compared to wild-type animals (Fig. 5l,
Supplementary Fig. S13a, b). These results suggest that Ras signalling may suppress oscillatory AIB activity. In the _let-60_ mutants, responses of AWC neurons to odour stimuli measured by
calcium imaging were identical to those of wild type, suggesting that Ras signalling is not involved in regulating depolarization of the AWC olfactory neurons themselves (Supplementary Fig.
S14). To investigate where Ras signalling acts to regulate AIB responses, we performed cell-specific rescue experiments. Expression of wild-type _let-60_ gene in AWC sensory neurons
significantly rescued the oscillatory AIB activity of the _let-60(lf)_ mutants (Fig. 5d, i, l). On the other hand, AIB-specific expression of _let-60_ did not (Fig. 5e, j, l). Furthermore,
overexpression of _let-60_ in AWC prevented the responses of AIB, in a manner similar to the _let-60(gf)_ mutants (Fig. 5f, k). These results demonstrate that Ras signalling in the AWC
sensory neurons affects the responses of downstream interneurons, which likely leads to abnormal olfactory behaviours. It was previously reported that the transmembrane guanylyl cyclase
GCY-28 and axonal diacylglycerol (DAG) signalling through the protein kinase C (PKC-1) regulate synaptic outputs of the AWC sensory neurons and the activity of AIB interneurons27. In
_gcy-28_ mutants, a calcium decrease in AIB after addition of butanone was not observed. Therefore, there is a possibility that both Ras signalling and GCY-28/PKC-1 singalling function in
the same pathway to regulate the output of AWC and the AIB activity. The putative null mutants of _gcy-28_, _gcy-28(tm2411)_, showed severer defects than the _let-60(n1046gf)_ and
_let-60(n2021lf)_ mutants in chemotaxis to isoamyl alcohol (Fig. 6a). _gcy-28(tm2411); let-60(n1046gf)_ and _gcy-28(tm2411); let-60(n2021lf)_ double mutants exhibited decreased chemotaxis as
much as _gcy-28(tm2411)_ single mutants (Fig. 6a). Thus, GCY-28 may act downstream of Ras signalling. It was previously reported that PKC-1 regulates synaptic transmission and neuropeptide
release28,29 and that PKC-1 acts downstream of or in parallel to GCY-28 to regulate AWC functions, since constitutive active form of PKC-1 rescued the chemotaxis defects of _gcy-28_
mutants27. We observed that expression of _pkc-1(gf)_ in AWC also significantly rescued the defects of _let-60_ mutants in chemotaxis to isoamyl alcohol (Fig. 6b, c). These results suggest
that PKC-1 acts downstream of or in parallel to Ras signalling to regulate output of AWC. DISCUSSION In this study, we performed live imaging of the activity of the major signalling protein,
Ras, in living animals. Raichu-Ras was previously characterized in cultured cell lines and activation of Ras was observed 15–30 min after stimulation with epidermal growth factors20. Also
in a single dendritic spine, Ca2+-dependent Ras activation was observed at the order of minutes, using Fras-F, another FRET-based indicator of Ras activation30. Therefore, our results
reveal, for the first time, fast kinetics of activation and inactivation of Ras at the order of seconds. In contrast to the activation of MAPK within 10 seconds of odour stimuli in AWC
neurons11, MAPK is activated 5 min after odour stimuli in interneurons critical for olfactory plasticity10 and SOS-1 is involved in the plasticity. Our results show that different Ras-GEFs
are used for olfactory reception or olfactory plasticity in the olfactory system in _C. elegans_, suggesting that different types of Ras-GEF may be used depending on the time scale of Ras
activation. A similar phenomenon is observed in T lymphocytes, where SOS and RasGRP function in parallel in the different time scales6,7. In response to increase in odour concentrations, Ras
is quickly activated and immediately inactivated. For klinotaxis, worms may have to sense changes in the odour concentration during each head swing25,31; during forward locomotion, each
head swing occurs over the course of a few seconds, which is consistent with the timescale of transient Ras activation. Thus, the dynamics of Ras activation may help to tune the
animal's quick response to changes in odour concentrations during klinotaxis. In _let-60(lf)_ mutants, oscillatory neural activity was observed in AIB. Oscillation of Ca2+
concentrations was previously observed in the AWC neurons of neuropeptide-deficient mutants24. Therefore, Ras signalling may regulate neuropeptide release to suppress oscillation of AIB
neural activity and regulate olfactory behaviours. It was previously reported that PKC-1 regulates neuropeptide release29 and our results suggest that GCY-28 and probably PKC-1 functions
downstream of Ras signalling. These results may support our idea that Ras signalling controls neuropeptide release of the AWC neurons. Our imaging analyses reveal that even if odour stimuli
were still present, Ras is quickly inactivated within a few second. On the other hand, the activity of MAPK may persist for longer time, because MAPK activation was observed after continuous
odour stimulation for 3 min22. Such distinct kinetics between Ras and MAPK suggest the possibility that quick inactivation of Ras may be also required for regulation of different signal
cascades from the MAPK pathway to control olfactory behaviours. Calcium imaging has been widely used in _C. elegans_ to examine neural activity in response to various stimuli32. The
activities of specific proteins, however, have not yet been observed in living animals of _C. elegans_. Our results provide a foundation for future probes and detection systems that will
allow monitoring of the activities of many proteins and their associated temporal regulation in _C. elegans_ and other organisms. METHODS STRAINS _C. elegans_ were cultured and maintained at
20°C under standard conditions33, except for the temperature sensitive strain _sos-1(cs41)_, which was cultured at 25°C. The NA22 _E. coli_ strain was used as a food source. Wild-type
animals were Bristol strain N2. Other strains used in this study included _tax-2(ks10) I_, _egl-30(ad806) I, gcy-28(tm2411) I, mpk-1(ga117) III_, _let-60(n1046gf) IV_, _let-60(n2021lf) IV_,
_sos-1(cs41) V_, _rgef-1(ok675) V_, _odr-3(n2150) V_ and _egl-8(n488)_ _V._ PLASMID CONSTRUCTION AND GERM-LINE TRANSFORMATION Raichu-Ras cDNA (kind gift from M. Matsuda) was inserted
downstream of the _odr-1_ promoter21. Mutant types of Raichu-Ras (m001, m102 and m103) were digested from pRaichu001, pRaichu102 and pRaichu103, respectively and inserted downstream of the
_odr-1_ promoter21. Sequence encoding yellow cameleon (YC) 3.6022 was connected to the _odr-1_ or _odr-2_ promoter21,34. _rgef-1_ cDNA was amplified by _C. elegans_ cDNA library. _rgef-1_,
_let-60_, _mpk-1_ and _pkc-1(gf)_ cDNA were inserted downstream of the _odr-1_ promoter, _npr-9_ promoter or the AIY-specific _ttx-3_ promoter for the expression in AWC, AIB or AIY,
respectively21,35,36. Germ-line transformations were performed using standard methods37. _lin-44p::GFP_ and _myo-3p::GFP_ were used as transformation markers. In Fig. 5, _odr-1p::let-60_ was
injected to the _let-60(lf)_ mutants at a concentration of 10 ng/μl for the rescue experiment and 50 ng/μl for the overexpression experiment. CELL-SPECIFIC KNOCKDOWN OF _MPK-1_ FUNCTIONS
The construction of transgenes to knock down the functions of _mpk-1_ gene in a specific neuron was performed as previously reported38. The targeted region of _mpk-1_ (1.4-kb genomic
sequences) was amplified with two primers Tf = 5′- gttcaattcaggggtgagga -3′ and Tr = 5′- caggattctgccctccatta -3′. _odr-1_ and AIY-specific _ttx-3_ promoters were used to express the
targeted gene in AWC and AIY neurons, respectively. RAS IMAGING AND ANALYSIS We used Raichu-Ras as probes. Animals that expressed Raichu-Ras in AWC neurons were glued on 4% agarose pads and
covered with physiologic buffer solution (80 mM NaCl, 5 mM KCl, 20 mM D-glucose, 10 mM HEPES, 5 mM MgCl2 and 1 mM CaCl2 at pH 7.2). Because signals from Raichu-Ras were weak, we did not use
a microfluidic device for the analysis owing to the associated noise with this system18,39; animals are able to move in the microfluidic device, which prevented identification of positive
signals. Therefore, the measurement of Ras activities after an odour removal was technically difficult. Isoamyl alcohol, which is sensed by AWC neurons, was diluted in ethanol and added to
the buffer as the odour stimulus. We confirmed that AWC olfactory neurons responded to the odorant by monitoring Ca2+ concentrations under the same conditions used for Ras imaging
(Supplementary Fig. S15). The final dilutions of the odorant were 5 × 10−4, 1 × 10−3 and 1.5 × 10−3 after the first, second and third stimulation, respectively. Room temperature was set at
20−23°C. Fluorescent images of Raichu-Ras were obtained using a Zeiss Axioplan 2 microscope equipped with 40× objective lens and ORCA-D2 digital camera (Hamamatsu). Images were collected at
an exposure of 200 msec. For long-term analyses of Raichu-Ras fluorescence (Fig. 3a, b, Supplementary Fig. S10), we used a 3CCD digital camera (C7780; Hamamatsu) and all images were
collected at an exposure of 500 msec. Similar results were obtained with these two systems (Supplementary Fig. S3). Time stacks of AWC cell bodies were captured and analyzed for the emission
ratio of YFP fluorescence to CFP fluorescence using Aqua Cosmos software (ver. 2.6, Hamamatsu). To distinguish weak signals representing Ras activity from noise, we excluded crosstalk
between the YFP and CFP emission spectra using a linear unmixing method (Multiband Imaging Software, Hamamatsu). The normalized ratio was calculated as the change in the YFP/CFP ratio
relative to the ratio value obtained 10 sec or 20 sec before the odour was applied. CALCIUM IMAGING AND STATISTICS To monitor the responses of AWC and AIB neurons, we generated
YC3.60-expressing lines using the _odr-1_ and _odr-2_ promoters21,22,34, respectively. Calcium imaging of AWC and AIB neurons were performed using a microfluidic device, as described
previously18,39, except for Supplementary Fig. S15 in which imaging was performed as described for Ras imaging. For experiments using a microfluidic device, we immobilized each animal by
trapping it in a microchannel, such that the nose of the animal was exposed to a flowing stream containing isoamyl alcohol (10−4 dilution) or odour-free solution, except for Supplementary
Fig. S9 where 10-5 and 10-7 dilutions of isoamyl alcohol were used. Optical equipment and conditions were similar to the other imaging experiments. CHEMOTAXIS AND OLFACTORY PLASTICITY ASSAYS
Chemotaxis assays were performed at 23°C as described previously17. Isoamyl alcohol was used as an attractive odorant at dilutions of 10−2, 10−3 and 10−4. Olfactory plasticity assays were
performed at 23°C as previously described10. When benzaldehyde was used for assays, the concentration was 10−4 for the initial exposure before the experiment and 10−2 for odorants spotted on
plates. The temperature sensitive strain _sos-1(cs41)_ts and wild-type control animals were cultured at 20°C or 25°C for 24 hrs before the assays and behavioural assays were performed at
23°C (Fig. 1a, Supplementary Fig. S7). _sos-1(cs41)_ts appeared nearly wild type at 20°C, but had strong larval lethal and vulvaless phenotypes at 25°C40. The chemotaxis index was calculated
as previously described10,17. MULTIWORM TRACKING SYSTEM We used a camera (Lw575M-IO; ARGO Corporation) and LED illumination (HPR-150SW; CCS) equipped with a digital power source (PD2-3024;
CCS). Images of 40−100 animals were captured from each assay plate. The interval between image captures was 500 msec and the recording time was 1 hour. Saved images were analyzed using
custom software—based on Lucam Capture software (ARGO Corporation)—that located the centroid of the animals. The design of the plate shown in Supplementary Fig. S11e was used without spotted
sodium azide. ANALYSIS OF TRACKING DATA Tracking data was analyzed essentially as described previously25. In a previous report25, data from immotile animals were discarded, whereas tracks
shorter than 1 mm were removed from the tracking data in this study. A sharp turn was defined as > 100° change in the direction of locomotion and consecutive sharp turns separated by less
than 3.18 sec were defined as a pirouette. Based on these criteria, locomotion was separated into pirouettes and other tracks (referred to as runs). Pirouettes were used to quantify
klinokinesis and runs were used to quantify klinotaxis. The direction to the odorant source relative to direction of locomotion and the curving rate were determined as shown in Supplementary
Fig. S11c, d. All angles were defined as positive for clockwise rotation. We calculated the pirouette index, basal pirouette frequency and weathervane index as previously described25 with
some modifications. During klinotaxis, worms respond to a chemical gradient perpendicular to the direction of locomotion25. On the other hand, during klinokinesis, worms respond to a
chemical gradient tangential to the direction of locomotion26. Therefore, the sine of bearing (θ) and cosine of bearing were used to quantify klinotaxis and klinokinesis, respectively. In
analyses of klinotaxis, the relationship between the sine θ and average curving rate was calculated (Supplementary Fig. S11c). All data points from the tracking data were classified into 10
groups according to the sine θ. The weathervane index was defined as the slope of the regression line and the average weathervane index for each assay was used for further analyses. In
analyses of klinokinesis, the relationship between the cosine θ and the probability of a pirouette was calculated (Supplementary Fig. S11d). All data points from the tracking data were
classified into 10 groups according to the cosine θ and the probability of pirouette initiation was plotted for the midpoint cosine θ in each group. The pirouette index was defined as the
negative slope of the regression line. The y intercept of this line is a measure of the basal pirouette frequency, because the direction of locomotion is perpendicular to the odour gradient
when cosine θ is 0. The average pirouette index and basal pirouette frequency in each assay are presented. FOURIER ANALYSIS The normalized ratio was calculated as the change in the YFP/CFP
ratio relative to the ratio value obtained 10 sec before the odour was applied. Data in the time domain (the normalized ratio trace _y_(_t_)) were transformed into the frequency domain
(_Y_(_t_)) using a formula; where _k_ = 0…N = 256. To estimate the total power contributed by oscillatory components in a band bounded by two frequencies _f_min and _f_max (0.03–1.0,
1.0–3.0, 3.0–5.0 Hz), we calculated integral of energy spectral density as REFERENCES * Williams, R. J. Signalling: basics and evolution. Acta Biochim Pol 51, 281–298 (2004). CAS PubMed
Google Scholar * Mitin, N., Rossman, K. L. & Der, C. J. Signaling interplay in Ras superfamily function. Curr Biol 15, R563–R574 (2005). Article CAS PubMed Google Scholar * Karnoub,
A. E. & Weinberg, R. A. Ras oncogenes: split personalities. Nat Rev Mol Cell Biol 9, 517–531 (2008). Article CAS PubMed PubMed Central Google Scholar * Ye, X. & Carew, T. J.
Small G protein signaling in neuronal plasticity and memory formation: the specific role of ras family proteins. Neuron 68, 340–361 (2010). Article CAS PubMed PubMed Central Google
Scholar * Malumbres, M. & Barbacid, M. RAS oncogenes: the first 30 years. Nat Rev Cancer 3, 459–465 (2003). Article CAS PubMed Google Scholar * Buday, L. & Downward, J. Many
faces of Ras activation. Biochim Biophys Acta 1786, 178–187 (2008). CAS PubMed Google Scholar * Das, J. et al. Digital signaling and hysteresis characterize ras activation in lymphoid
cells. Cell 136, 337–351 (2009). Article CAS PubMed PubMed Central Google Scholar * Omerovic, J., Laude, A. J. & Prior, I. A. Ras proteins: paradigms for compartmentalised and
isoform-specific signalling. Cell Mol Life Sci 64, 2575–2589 (2007). Article CAS PubMed PubMed Central Google Scholar * Sundaram, M. V. RTK/Ras/MAPK signaling. WormBook. 2006 Feb 11,
1–19 (2006). Google Scholar * Hirotsu, T. & Iino, Y. Neural circuit-dependent odor adaptation in C. elegans is regulated by the Ras-MAPK pathway. Genes Cells 10, 517–530 (2005). Article
CAS PubMed Google Scholar * Hirotsu, T., Saeki, S., Yamamoto, M. & Iino, Y. The Ras-MAPK pathway is important for olfaction in Caenorhabditis elegans. Nature 404, 289–293 (2000).
Article CAS ADS PubMed Google Scholar * Roayaie, K., Crump, J. G., Sagasti, A. & Bargmann, C. I. The G alpha protein ODR-3 mediates olfactory and nociceptive function and controls
cilium morphogenesis in C. elegans olfactory neurons. Neuron 20, 55–67 (1998). Article CAS PubMed Google Scholar * Birnby, D. A. et al. A transmembrane guanylyl cyclase (DAF-11) and
Hsp90 (DAF-21) regulate a common set of chemosensory behaviors in caenorhabditis elegans. Genetics 155, 85–104 (2000). CAS PubMed PubMed Central Google Scholar * L'Etoile, N. D.
& Bargmann, C. I. Olfaction and odor discrimination are mediated by the C. elegans guanylyl cyclase ODR-1. Neuron 25, 575–586 (2000). Article CAS PubMed Google Scholar * Komatsu, H.,
Mori, I., Rhee, J. S., Akaike, N. & Ohshima, Y. Mutations in a cyclic nucleotide-gated channel lead to abnormal thermosensation and chemosensation in C. elegans. Neuron 17, 707–718
(1996). Article CAS PubMed Google Scholar * Coburn, C. M. & Bargmann, C. I. A putative cyclic nucleotide-gated channel is required for sensory development and function in C. elegans.
Neuron 17, 695–706 (1996). Article CAS PubMed Google Scholar * Bargmann, C. I., Hartwieg, E. & Horvitz, H. R. Odorant-selective genes and neurons mediate olfaction in C. elegans.
Cell 74, 515–527 (1993). Article CAS PubMed Google Scholar * Chalasani, S. H. et al. Dissecting a circuit for olfactory behaviour in Caenorhabditis elegans. Nature 450, 63–70 (2007).
Article CAS ADS PubMed Google Scholar * Beitel, G. J., Clark, S. G. & Horvitz, H. R. Caenorhabditis elegans ras gene let-60 acts as a switch in the pathway of vulval induction.
Nature 348, 503–509 (1990). Article CAS ADS PubMed Google Scholar * Mochizuki, N. et al. Spatio-temporal images of growth-factor-induced activation of Ras and Rap1. Nature 411,
1065–1068 (2001). Article CAS ADS PubMed Google Scholar * Yu, S., Avery, L., Baude, E. & Garbers, D. L. Guanylyl cyclase expression in specific sensory neurons: a new family of
chemosensory receptors. Proc Natl Acad Sci U S A 94, 3384–3387 (1997). Article CAS ADS PubMed PubMed Central Google Scholar * Chen, L., Fu, Y., Ren, M., Xiao, B. & Rubin, C. S. A
RasGRP, C. elegans RGEF-1b, couples external stimuli to behavior by activating LET-60 (Ras) in sensory neurons. Neuron 70, 51–65 (2011). Article CAS PubMed PubMed Central Google Scholar
* Lackner, M. R., Kornfeld, K., Miller, L. M., Horvitz, H. R. & Kim, S. K. A MAP kinase homolog, mpk-1, is involved in ras-mediated induction of vulval cell fates in Caenorhabditis
elegans. Genes Dev 8, 160–173 (1994). Article CAS PubMed Google Scholar * Chalasani, S. H. et al. Neuropeptide feedback modifies odor-evoked dynamics in Caenorhabditis elegans olfactory
neurons. Nat Neurosci 13, 615–621 (2010). Article CAS PubMed PubMed Central Google Scholar * Iino, Y. & Yoshida, K. Parallel use of two behavioral mechanisms for chemotaxis in
Caenorhabditis elegans. J Neurosci 29, 5370–5380 (2009). Article CAS PubMed PubMed Central Google Scholar * Pierce-Shimomura, J. T., Morse, T. M. & Lockery, S. R. The fundamental
role of pirouettes in Caenorhabditis elegans chemotaxis. J Neurosci 19, 9557–9569 (1999). Article CAS PubMed PubMed Central Google Scholar * Tsunozaki, M., Chalasani, S. H. &
Bargmann, C. I. A behavioral switch: cGMP and PKC signaling in olfactory neurons reverses odor preference in C. elegans. Neuron 59, 959–971 (2008). Article CAS PubMed PubMed Central
Google Scholar * Sieburth, D. et al. Systematic analysis of genes required for synapse structure and function. Nature 436, 510–517 (2005). Article CAS ADS PubMed Google Scholar *
Sieburth, D., Madison, J. M. & Kaplan, J. M. PKC-1 regulates secretion of neuropeptides. Nat Neurosci 10, 49–57 (2007). Article CAS PubMed Google Scholar * Harvey, C. D., Yasuda, R.,
Zhong, H. & Svoboda, K. The spread of Ras activity triggered by activation of a single dendritic spine. Science 321, 136–140 (2008). Article CAS ADS PubMed PubMed Central Google
Scholar * Izquierdo, E. J. & Lockery, S. R. Evolution and analysis of minimal neural circuits for klinotaxis in Caenorhabditis elegans. J Neurosci 30, 12908–12917 (2010). Article CAS
PubMed PubMed Central Google Scholar * Dittman, J. Worm watching: imaging nervous system structure and function in Caenorhabditis elegans. Adv Genet. 65, 39–78 (2009). Article CAS
PubMed Google Scholar * Brenner, S. The genetics of Caenorhabditis elegans. Genetics 77, 71–94 (1974). CAS PubMed PubMed Central Google Scholar * Chou, J. H., Bargmann, C. I. &
Sengupta, P. The Caenorhabditis elegans odr-2 gene encodes a novel Ly-6-related protein required for olfaction. Genetics 157, 211–224 (2001). CAS PubMed PubMed Central Google Scholar *
Bendena, W. G. et al. A Caenorhabditis elegans allatostatin/galanin-like receptor NPR-9 inhibits local search behavior in response to feeding cues. Proc Natl Acad Sci U S A 105, 1339–1342
(2008). Article CAS ADS PubMed PubMed Central Google Scholar * Wenick, A. S. & Hobert, O. Genomic cis-regulatory architecture and trans-acting regulators of a single
interneuron-specific gene battery in C. elegans. Dev Cell 6, 757–770 (2004). Article CAS PubMed Google Scholar * Mello, C. C., Kramer, J. M., Stinchcomb, D. & Ambros, V. Efficient
gene transfer in C.elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J 10, 3959–3970 (1991). Article CAS PubMed PubMed Central Google Scholar *
Esposito, G., Di Schiavi, E., Bergamasco, C. & Bazzicalupo, P. Efficient and cell specific knock-down of gene function in targeted C. elegans neurons. Gene 395, 170–176 (2007). Article
CAS PubMed Google Scholar * Chronis, N., Zimmer, M. & Bargmann, C. I. Microfluidics for in vivo imaging of neuronal and behavioral activity in Caenorhabditis elegans. Nat Methods 4,
727–731 (2007). Article CAS PubMed Google Scholar * Rocheleau, C. E. et al. A lin-45 raf enhancer screen identifies eor-1, eor-2 and unusual alleles of Ras pathway genes in
Caenorhabditis elegans. Genetics 161, 121–131 (2002). CAS PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS We thank the _Caenorhabditis_ Genetic Center (CGC) for
the strains, M. Matsuda for series of Raichu-Ras probes, A. Miyawaki and T. Nagai for YC3.60, H. Ohno for _mpk-1_ cDNA, C. Bargmann for _pkc-1(gf)_ cDNA, T. Teramoto for imaging constructs
and strains, R. Iritani for the Fourier analysis, H. Tachida for useful advice and members of our laboratory for discussion. This research was supported by Grant-in-aid for Young Scientists
(A), JSPS, Japan. AUTHOR INFORMATION Author notes * Uozumi Takayuki and Hirotsu Takaaki contributed equally to this work. AUTHORS AND AFFILIATIONS * Department of Biology, Graduate school of
Sciences, Kyushu University, Fukuoka, 812-8581, Japan Takayuki Uozumi, Takaaki Hirotsu, Ryuji Yamada, Akiya Suzuki, Gun Taniguchi & Takeshi Ishihara * Department of Biophysics and
Biochemistry, Graduate School of Sciences, The University of Tokyo, Tokyo, 113-0032, Japan Kazushi Yoshida & Yuichi Iino Authors * Takayuki Uozumi View author publications You can also
search for this author inPubMed Google Scholar * Takaaki Hirotsu View author publications You can also search for this author inPubMed Google Scholar * Kazushi Yoshida View author
publications You can also search for this author inPubMed Google Scholar * Ryuji Yamada View author publications You can also search for this author inPubMed Google Scholar * Akiya Suzuki
View author publications You can also search for this author inPubMed Google Scholar * Gun Taniguchi View author publications You can also search for this author inPubMed Google Scholar *
Yuichi Iino View author publications You can also search for this author inPubMed Google Scholar * Takeshi Ishihara View author publications You can also search for this author inPubMed
Google Scholar CONTRIBUTIONS T. U. performed the imaging experiments, analyzed the data and wrote the paper; T. H. designed the study, performed the behavioural analyses and wrote the paper;
R. Y. and A. S. performed the imaging analyses; G. T. performed epistasis analyses; K. Y. and Y. I. performed the behavioural experiments and analyzed the data; and T. I. designed the
experiments and developed some of the analytical tools. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. ELECTRONIC SUPPLEMENTARY MATERIAL
SUPPLEMENTARY INFORMATION Supplementary Movie S1 SUPPLEMENTARY INFORMATION Supplementary Information RIGHTS AND PERMISSIONS This work is licensed under a Creative Commons
Attribution-NonCommercial-ShareALike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/ Reprints and permissions ABOUT THIS ARTICLE
CITE THIS ARTICLE Uozumi, T., Hirotsu, T., Yoshida, K. _et al._ Temporally-regulated quick activation and inactivation of Ras is important for olfactory behaviour. _Sci Rep_ 2, 500 (2012).
https://doi.org/10.1038/srep00500 Download citation * Received: 23 January 2012 * Accepted: 22 June 2012 * Published: 09 July 2012 * DOI: https://doi.org/10.1038/srep00500 SHARE THIS ARTICLE
Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard
Provided by the Springer Nature SharedIt content-sharing initiative