Detection of preperimetric glaucoma using bruch membrane opening, neural canal and posterior pole asymmetry analysis of optical coherence tomography
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT We analysed retinal nerve fibre layer (RNFL) defects in eyes with normal circumpapillary RNFL (cpRNFL) thickness using posterior pole asymmetry analysis (PPAA) and investigated the
parameters of Bruch membrane opening (BMO) and neural canals using enhanced depth imaging spectral domain optical coherence tomography (EDI-SDOCT). A total of 112 preperimetric glaucomatous
eyes of 92 patients were examined to obtain cpRNFL thickness using SD-OCT. Posterior pole asymmetry analysis (PPAA) and central cross-sectional images of the optic nerve head (ONH) were
obtained using EDI-SDOCT. Minimal and horizontal distances between the BMO and ONH surfaces (BMOM, BMOH) and the terminal of retinal pigment epithelium (RPE) and ONH surfaces (RPEM, RPEH)
were measured. The distribution of the absolute black cells in PPAA was more concentrated in eyes with “U”-shaped neural canals (p < 0.0001). The area under the receiver operating
characteristic curve of the ratio of RPEM to RPEH (RPE-R, 0.771 ± 0.08) was significantly larger than the ratio of BMOM to BMOH (BMO-R, 0.719 ± 0.009) for PPAA results. A U-shaped neural
canal, lower ratio of RPEM to RPEH, and lower ratio of BMOM to BMOH were considered early indicators of RNFL defects in preperimetric glaucomatous eyes with normal cpRNFL. SIMILAR CONTENT
BEING VIEWED BY OTHERS QUANTIFICATION OF PERIPAPILLARY NERVE FIBRE ELEVATION AND ITS ASSOCIATION WITH AXIAL LENGTH, OPTIC DISC TILT, AND PARAPAPILLARY ATROPHY AREA IN YOUNG, HEALTHY EYES
Article 15 November 2023 THE INFLUENCE OF DIFFERENT INTRAOCULAR PRESSURE ON LAMINA CRIBROSA PARAMETERS IN GLAUCOMA AND THE RELATION CLINICAL IMPLICATION Article Open access 07 May 2021
THICKNESS OF RETINAL PIGMENT EPITHELIUM–BRUCH’S MEMBRANE COMPLEX IN ADULT CHINESE USING OPTICAL COHERENCE TOMOGRAPHY Article 20 January 2022 INTRODUCTION Glaucoma is an optic neuropathy with
chronic neurodegenerative changes and progressive degeneration of the retinal ganglion cell (RGC) axons1, leading to the loss of visual field. The detection of early stage glaucoma, i.e.,
preperimetric glaucoma, has attracted increasing attention. However, the identification of preperimetric glaucoma via retinal nerve fibre layer defects (RNFLDs) remains controversial in
clinical practice. Red-free reflectance imaging is used in the detection of RNFLD with high accuracy2, but the data cannot be analysed quantitatively, and the image quality is affected by
many factors, such as cataract, leading to signal attenuation. Spectral domain optical coherence tomography (SD-OCT) is a relatively new imaging approach for the diagnosis and management of
glaucoma3. Unfortunately, previous studies demonstrated that measurement of the circumpapillary retinal nerve fibre layer (cpRNFL) using SD-OCT provided unsatisfactory diagnostic capability
and exhibited only moderate sensitivity in the detection of some cases with early RNFLD4,5 despite of its potential to recognize these defects. Normal cpRNFL was observed in many eyes with
preperimetric glaucoma, supporting the development of new techniques and more reliable criteria for diagnosis. The focus for the diagnosis of preperimetric glaucoma has recently moved from
the peripheral retina to the macula. Glaucomatous damage involves a progressive loss of retina ganglion cells (RGCs). Therefore, observations of macular changes were added for structural
assessments of glaucoma. RGCs are thickest in the perimacular area, and RGCs and RNFL constitute 30% to 35% of the retinal thickness in this region6. Therefore, damage in the RGCs is more
readily detected in the macula than the peripheral retina. Early glaucomatous defects are generally localized to either side of the horizontal meridian, which allows the possible evaluation
of asymmetry in hemifield macular thickness. This asymmetry has served as an early indicator of glaucomatous structural damage7. Recent studies found a significant reduction in the ratio of
localized RNFL thickness to total retinal thickness during the early stages of glaucoma8, and the localized RNFL thickness exhibits a statistically significant structure-function association
with the macular visual field (VF)9. Seo, _et al._10 and Asrani _et al._11 introduced a new method, termed as posterior pole asymmetry analysis (PPAA), using SD-OCT to test the difference
between upper and lower macular thickness and detect localized RNFLD with higher sensitivity and specificity than the cpRNFL thickness10. They reported that the retinal thickness maps
acquired through SD-OCT identified the presence of visible localized RNFLD and detected the thinning of RNFL, which is not otherwise detectable using stereo-photographic assessments12.
However, these PPAA studies were generally conducted in abnormal cpRNFL eyes instead of normal cpRNFL eyes. In addition to RNFL thickness, three anatomical structures of the optic nerve head
(ONH) are of great concern in the discrimination of glaucomatous eyes, particularly preperimetric glaucoma, from healthy eyes. First, the neural canal opening (NCO), including the
termination of the retinal pigment epithelium (RPE)/Bruch’s membrane (BM) complex, is not likely to change substantially with glaucoma progression13. Second, the BM opening (BMO), which is
the internal entrance to the neural canal and an important structure of the ONH, is likely the true optic disc margin, and it was proposed as a stable zero reference plane for ONH
quantification14,15. Third, anterior laminar displacement of lamina cribrosa (LC) tissue occurs after glaucoma surgery16. The LC is not a static structure, and it responds to intraocular
pressure (IOP) changes. Once IOP rises in glaucomatous eyes, a cup excavation increase will be observed, primarily at the expense of prelaminar tissue thinning. LC thickness is significantly
greater in normal subjects than glaucoma patients. The degree of thinning significantly correlates with glaucoma severity and increases with glaucomatous damage. Normal LC morphology in
enhanced depth imaging SD-OCT (EDI-SDOCT) exhibits a “W” shape without an enlarged neural canal, but a “U” shape is observed when IOP increases, which indicates a thinner LC and enlarged
neural canal. Therefore, LC plays a critical role in the pathogenesis of glaucoma, and EDI-SDOCT reveals the _in vivo_ features of LC17. Agoumi _et al._18 used manual delineation to evaluate
the laminar and prelaminar surfaces and demonstrated changes in the LC and prelaminar tissue after IOP elevation. However, the correlation between the morphology of ONH and PPAA has not
been assessed in preperimetric glaucomatous eyes, particularly eyes with normal cpRNFL results. Therefore, we investigated subjects with preperimetric glaucomatous eyes and normal cpRNFL
thickness. Eyes also exhibited typical wedge-shaped defects in red-free reflection and absolute black cells in PPAA. We further analysed PPAA patterns and the correlation between PPAA
results and ONH morphology and also present new parameters for the diagnosis of glaucoma in patients with normal cpRNFL results. RESULTS GENERAL INFORMATION This study included 112 eyes of
92 Chinese (northern Han race) patients with normal cpRNFL thickness from 28 males and 64 females with a mean age of 56.5 years (range: 19–77 years). The spherical equivalent (SE) was −0.75
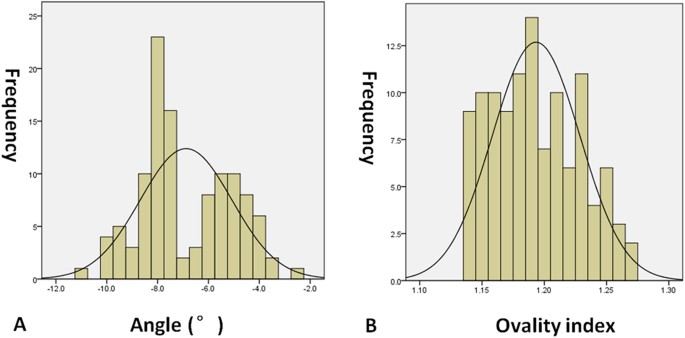
diopters (range: −1.75– + 1.50 diopters) and the axial length was 23.73 mm (range: 22.58–24.35 mm). In addition, the angle between the fovea and ONH centre relative to the horizontal axis
was −7.5°(range: −11.0°–2.4°), and the ovality index was 1.19 (range: 1.14–1.27) (Fig. 1). Moreover, the outlook of ONH was without torsion, and staphyloma. Besides, EDI-SDOCT identified
that 60 of the 112 eyes exhibited “U”-shaped neural canals, and the remaining 52 eyes exhibited “W”-shaped neural canals (Table 1). THE TOPOGRAPHY OF PPAA Three additional zones were added
in each hemifield (Zones 6, 7, and 8) compared with previous studies of PPAA (Fig. 2). This addition may cover the entire retinal fibre region related to glaucoma injury. Zone 7 represents
the papillomacular bundles. Zone 6 adds peripheral retinal nerve fibre information from the nasal and temporal sides of the retina. Zone 8 supplies peripheral retinal nerve fibre information
from the temporal retina. The present study found that the absolute black cells were primarily concentrated in inferior Zones 4, 5, and 6 (45.8%) and superior Zones 5, 6, and 7 (38.9%).
Zones 6, 7, and 8 contained 8.8%, 24.2%, and 2.2% black cells, respectively (Fig. 3). Zone 7 is very vulnerable to the glaucoma injury process. A total of 288 absolute black cells were
counted in PPAA maps, and these cells concentrated in inferior Zones 4, 5, and 6 (45.8%) and superior Zones 5, 6, and 7 (38.9%) (Figs 2 and 3, respectively). PPAA (+) was identified in 64
eyes (36, 12 and 16 of these eyes contained 2, 3 and 4 consecutive absolute black cells, respectively). Fifty-two eyes exhibited “W”-shaped neural canals, and the other 60 eyes exhibited
“U”-shaped neural canals (Fig. 4). Twenty, 8 and 4 eyes with “W”-shaped neural canals exhibited 1, 2 and 3 consecutive absolute black cells, respectively. The remaining 20 eyes did not
contain any absolute black cells. Eight, 28, 8 and 16 eyes with “U”-shaped neural canal exhibited 1, 2, 3 and 4 absolute black cells, respectively. The absolute black cells in PPAA were
obviously more concentrated in the eyes with “U”-shaped neural canals (r = 0.653, p < 0.0001). There was a significant positive correlation between the PPAA results and the morphology of
the neural canal (“U” or “W” shaped) (r = 0.641, p < 0.0001). We measured the minimal and horizontal distances between the terminal of the RPE and ONH surfaces (RPEM and RPEH,
respectively) and the BMO and ONH surfaces (BMOM and BMOH, respectively) to more quantitatively assess the NCO in these subjects (Fig. 5). The ratios of BMOM to BMOH (BMO-R) and RPEM to RPEH
(RPE-R) were calculated. The results demonstrated that the AUROC of RPE-R (0.771 ± 0.08) was significantly larger than the BMO-R (0.719 ± 0.009) for PPAA (+) results (Fig. 6 & Table 1).
RPEM also exhibited a significant relationship with BMOM (r = 0.607, p = 0.001 < 0.05). There was a significant difference in RPEM between eyes with “U”-shaped and “W”-shaped neural
canals (Z = 2.004, p = 0.045 < 0.05). The difference in BMOM between eyes with the “U”-shaped and “W”-shaped neural canals was not statistically significant (Z = 1.244, p = 0.214 >
0.05). DISCUSSION VF testing is an important traditional method for the diagnosis of glaucoma, but the recognition of RNFLD in preperimetric glaucoma or the preglaucomatous stage has
attracted increasing attention since the finding that perimetrically normal hemifields of glaucomatous eyes exhibit significantly lower macular GCC and cpRNFL thicknesses than the
corresponding retinal regions of healthy eyes19. However, cpRNFL may not be a reliable indicator because the thickness of cpRNFL is normal in some eyes with preperimetric glaucoma10. The
present study assumed that the subjects with typical wedge-shaped defects in red-free reflection imaging and the presence of absolute black cells in PPAA had glaucoma. Therefore, we
investigated the characteristics of PPAA in eyes with preperimetric glaucoma and normal cpRNFL thickness. These eyes exhibited the typical wedge-shaped defects in red-free reflection and
absolute black cells in PPAA, which are indicators of preperimetric glaucoma7. We also analysed the ONH morphology using EDI-SDOCT and introduced a new parameter, the RPE-R, as a
complementary method to BMO measurements for the diagnosis of early glaucoma. The measurement of normal retinal thickness was first described in the 1990s20, and its loss is associated with
glaucoma21. The idea was primarily based on the progressive loss of RGCs during glaucomatous damage22. The symmetric distribution of retinal nerve fibres in reference to the fovea-disc axis
is perturbed as glaucoma develops. Therefore, the glaucoma hemifield test in VF becomes a sensitive indicator of early glaucoma23. Asymmetric retinal thickness is a potentially valuable
structural testing target for the diagnosis of glaucoma. Bagga _et al._24 demonstrated that macular thickness asymmetry exhibited an AUROC of 0.76 to 0.84, with the potential to detect
glaucomatous damage. The present study found that the distribution of absolute black cells in eight zones primarily centred on inferior Zones 4, 5, and 6 and superior Zones 5, 6, and 7,
which coincided with RGC distribution. One explanation may be that the nasal to the macula areas convey fibres from the retina, both nasally and temporally, to the macula, whereas the
temporal retina contains only the temporal fibres, resulting in a worse performance than the nasal area. The superior and inferior arcuate nerve fibres are also vulnerable to early
glaucomatous changes7. Macular inner retinal layer thickness exhibits a significantly larger AUROC than cpRNFL thickness25. The subjects in the present study had a normal cpRNFL thickness,
suggesting that injury of the RNFL in preperimetric glaucoma may occur at an earlier time in the macula than the circumpapillary area, which may be less sensitive7. Chauhan _et al._26
recently concluded that the clinically visible disc margin is an unreliable outer border of rim tissue compared to SD-OCT-based approaches for rim assessments because of the clinically and
photographically invisible extensions of the BM. In contrast, BMO minimum rim width quantifies the neuroretinal rim from the true anatomical outer border and accounts for its variable
trajectory at the point of measurement, and this measurement is also attributed to the introduction of the SD-OCT approach27. The present study further applied EDI-SDOCT to measure the
minimal and horizontal distances between the BMO and ONH surfaces (i.e., BMOM and BMOH) and found that the ratio of BMOM to BMOH, termed as the BMO-R, in PPAA (+) eyes was significantly
smaller than PPAA (−) eyes, which corresponded to optic nerve atrophy in our examinations. Measurements of the BMO cross-sectional diameter and the mean distance from the BMO plane to the
anterior surface of the collagenous LC on the SD-OCT profile were strongly associated with matched histological measurements28. Therefore, BMO has become more popular for the assessment of
ONH related-diseases29,30,31. We performed more quantitative assessments of these structures than previous studies. Notably, our study is the first study to measure and assess the RPE-R
(i.e., the ratio of RPEM to RPEH). We further found that the AUROC of RPE-R (0.771 ± 0.08) was significantly larger than the BMO-R (0.719 ± 0.009) for PPAA (+) results, suggesting that the
RPE-R may be a more sensitive and reliable parameter than traditional BMO-R for PPAA (+) and RNFLD detection. Therefore, RPE-R may serve as a reliable marker for the early diagnosis of
glaucoma. We also found that the morphology of the neural canal correlated with the PPAA results. Notably, eyes with “U”-shaped neural canals contained more absolute black cells. The classic
posterior LC bowing and compression and excavation of the scleral canal wall beneath the BMO were observed in moderately or severely damaged glaucomatous eyes32. Downs _et al._15 recently
reported that profound deformations of the neural canal and the anterior-most aspect of the subarachnoid space architecture are observed at the onset of ONH surface changes in early
experimental glaucomatous eyes of young adult monkeys. Nicholas _et al._33 also found that the neuroretinal rim decreased and anterior LC surface depth increased significantly, but no change
in RNFL thickness was detected. Similarly and additionally, we demonstrated these changes in human eyes with PPAA (+). This study also has some limitations because of its retrospective
design. We only analysed the absolute black cells in PPAA instead of calculating the varying levels of absolute black cells, and also only assessed the morphology of ONH in the vertical OCT
profile. In addition, we did not analyse the correlation of ovality index (the tilt and shape of the optic nerve)34 with PPAA results and LC shape. All these will be improved in further
researches. In conclusion, we found that the distribution of absolute black cells in PPAA coincided with RGC distribution. There was a significant positive relationship between PPAA results
and the morphology of the neural canal (“U” or “W” shaped). Eyes with a “U”-shaped neural canal contained more absolute black cells. Our study is the first study to measure and assess RPE-R,
refering to the ratio of RPEM to RPEH. RPE-R may be a more sensitive and reliable parameter than traditional BMO-R for PPAA (+) and RNFLD detection. Therefore, RPE-R may serve as a reliable
marker for the early diagnosis of glaucoma. Therefore, a “U”-shaped neural canal and lower BMO-R and RPE-R are proposed as novel early indicators of RNFLD in these glaucoma cases. METHODS
PATIENTS This retrospective study included patients who attended the Department of Ophthalmology - glaucomatous outpatient clinic at China Medical University. All patients had undergone
stereoscopic disc examination, IOP measurement, refractive status (SE was calculated as the sum of the spherical plus half of the cylindrical error), A scan ultrasonography (axial length;
AVISO, Quantel medical, France), visual field testing (central 30 degrees; Zeiss 750i Humphrey VF automated perimetry, Germany), and red-free reflection imaging (Spectralis HRA + OCT;
Heidelberg Engineering, Heidelberg, Germany). Subjects also underwent SD-OCT (Spectralis HRA + OCT; Heidelberg Engineering, Heidelberg, Germany) examination to obtain cpRNFL thickness, PPAA
and central vertical sectional imaging of ONH with EDI function. The raw images of each line scan in PPAA have been reviewed for the absence of artifacts. Two experienced and qualified
reviewers (R.H. and X.L.M) separately assessed all red-free images to identify the RNFL losses according to the study of Seo _et al._10. Discrepancies were referred to fundus specialists
(K.Y. and R.G.) for final determination. At the same time, the ovality index defined as the ratio of the longest diameter to the shortest diameter of the optic disc was also measured in
red-free images according to the study of Lee _et al._34. Subjects with normal cpRNFL thickness results defined as the global normal average measurement of the cpRNFL for each section
(including temporal-superior (45°–90°), nasal-superior (90°–135°), nasal (135°–225°), nasal-inferior (225°–270°), temporal-inferior (270°–315°), and temporal (315°–45°)), typical
wedge-shaped defects in red-free reflection imaging and any absolute black cells in PPAA were included in our study, and they did not receive any IOP-lowering treatment. Subjects were
excluded if they had typical glaucomatous VF changes, IOP > 21 mmHg and a glaucomatous optic disc that exhibited increased cupping (vertical cup–disc ratio>0.6), vertical cup–disc
ratio asymmetry of >0.2 between the two eyes, history of high myopia or other diseases affecting retinal thickness and ONH assessment, such as ischemic optic neuropathy, age-related
macular degeneration, epimacular membrane or macular oedema. The study adhered to the tenets of the Declaration of Helsinki, and the Medical Research Ethics Committee of China Medical
University approved the study. Informed consent was obtained from all subjects. PPAA MEASUREMENT PPAA was performed in the macular 20° area of each eye using a 30° × 25° OCT volume scan with
a colour scale representation of topographic retinal thickness. The posterior pole map provided information on the retinal thickness value of 64 (8 × 8) cells within each cell. The angle
between the fovea and ONH centre relative to the horizontal axis was measured by FoDi correction line function of the Heidelberg Eye explorer software (version 1.5.12.0, Heidelberg
Engineering, Heidelberg, Germany) automatically, simultaneously with cpRNFL detecting, and the negative angle was defined as the fovea was located below the level of the centre of the ONH,
according to the study of Chauhan _et al._26. The 8 × 8 grid was positioned symmetrically to the fovea-disc axis with the central point of the grid on the fovea, which was used for the
inter-hemisphere comparisons.7 PPAA also provided a corresponding cell-to-cell comparison between hemispheres within the central 20°, and the differences are presented using a grey scale.
Absolute black cells in PPAA refer to a retinal thickness difference of more than or equal to 30 μm, and this difference was recorded because it is difficult to clearly discern the values
using a grey scale other than absolute black. Seo _et al._10 referred to eyes with 2, 3, or 4 consecutive absolute black cells in PPAA as PPAA (+) and eyes with sporadic absolute black cells
in PPAA as PPAA (−). Eight zones in the macular thickness map were defined. Each zone included reciprocal areas in the superior and inferior hemifield. We defined these zones according to
Um _et al._7, and three more zones were added in each hemifield. ONH MEASUREMENT ONH imaging was acquired using EDI-SDOCT. We defined the morphology of the neural canal including the
anterior lamina cribrosa surface16,33,35 and excavation of the peripapillary sclera as “W” or “U” shaped according to the studies of Lee _et al._36 and Rebolleda _et al._37. Several
experimental studies of enucleated human eyes also reported that posterior displacement was greatest in the middle of the lamina when an elevated IOP was applied, and the lamina assumed a U
shape38. The minimal and horizontal distances between the BMO and ONH surfaces (BMOM and BMOH, respectively)27 were measured in the superior and inferior parts, respectively, using
Heidelberg Eye explorer software (version 1.5.12.0, Heidelberg Engineering, Heidelberg, Germany) as the study of Chauhan _et al._26. The terminal of RPE was used in Heidelberg Retina
Tomograph III and Optovue RTVue OCT38 as part of the NCO, and we measured the minimal and horizontal distances between the terminal of RPE and ONH surfaces (RPEM and RPEH, respectively)
similarly. The ratios of BMOM and BMOH (BMO-R) and RPEM and RPEH (RPE-R) were calculated. A predictable pattern of axonal loss underlies early glaucomatous VF loss, which is ascribed to the
inferior and superior poles of the ONH. Therefore, we chose the central cross-sectional image of ONH on OCT profile as a reference39,40. STATISTICAL ANALYSIS All analyses were performed
using SPSS version 19.0 (Inc., Chicago, IL). The data were expressed as medians (Min-Max). Spearman’s rank correlation test was performed on the relationship between RPE-R and BMO-R and the
relationship between the absolute black cells in PPAA and neural canal morphology. The area under the receiver operating characteristic curve (AUROC) was calculated to detect the diagnostic
ability of RPE-R and BMO-R for PPAA. A probability (p) value of less than 0.05 was considered statistically significant. ADDITIONAL INFORMATION HOW TO CITE THIS ARTICLE: Hua, R. _et al._
Detection of preperimetric glaucoma using Bruch membrane opening, neural canal and posterior pole asymmetry analysis of optical coherence tomography. _Sci. Rep._ 6, 21743; doi:
10.1038/srep21743 (2016). REFERENCES * Schlamp, C. L., Li, Y., Dietz, J. A., Janssen, K. T. & Nickells, R. W. Progressive ganglion cell loss and optic nerve degeneration in DBA/2J mice
is variable and asymmetric. _BMC Neurosci_ 7, 66 (2006). Article PubMed PubMed Central Google Scholar * Suh, M. H., Kim, D. M., Kim, Y. K., Kim, T. W. & Park, K. H. Patterns of
progression of localized retinal nerve fibre layer defect on red-free fundus photographs in normal-tension glaucoma. _Eye (Lond)_ 24, 857–863 (2010). Article CAS Google Scholar * Aref, A.
A. & Budenz, D. L. Spectral domain optical coherence tomography in the diagnosis and management of glaucoma. _Ophthalmic Surg Lasers Imaging_ 41, S15–27 (2010). Article PubMed Google
Scholar * Kim, T. W., Park, U. C., Park, K. H. & Kim, D. M. Ability of Stratus OCT to identify localized retinal nerve fiber layer defects in patients with normal standard automated
perimetry results. _Invest Ophthalmol Vis Sci_ 48, 1635–1641 (2007). Article PubMed Google Scholar * Jeoung, J. W., Park, K. H., Kim, T. W., Khwarg, S. I. & Kim, D. M. Diagnostic
ability of optical coherence tomography with a normative database to detect localized retinal nerve fiber layer defects. _Ophthalmology_ 112, 2157–2163 (2005). Article PubMed Google
Scholar * Zeimer, R., Asrani, S., Zou, S., Quigley, H. & Jampel, H. Quantitative detection of glaucomatous damage at the posterior pole by retinal thickness mapping. A pilot study.
_Ophthalmology_ 105, 224–231 (1998). Article CAS PubMed Google Scholar * Um, T. W. et al. Asymmetry in hemifield macular thickness as an early indicator of glaucomatous change. _Invest
Ophthalmol Vis Sci_ 53, 1139–1144 (2012). Article PubMed PubMed Central Google Scholar * Kita, Y. et al. Ability of Optical Coherence Tomography-determined Ganglion Cell Complex
Thickness to Total Retinal Thickness Ratio to Diagnose Glaucoma. _J Glaucoma_ 22, 757–762 (2013). Article PubMed Google Scholar * Na, J. H., Kook, M. S., Lee, Y. & Baek, S.
Structure-function relationship of the macular visual field sensitivity and the ganglion cell complex thickness in glaucoma. _Invest Ophthalmol Vis Sci_ 53, 5044–5051 (2012). Article PubMed
Google Scholar * Seo, J. H. et al. Detection of Localized Retinal Nerve Fiber Layer Defects with Posterior Pole Asymmetry Analysis of Spectral Domain Optical Coherence Tomography. _Invest
Ophthalmol Vis Sci_ 53, 4347–4353 (2012). Article PubMed Google Scholar * Sanjay Asrani, Jullia A. Rosdahl & R. Rand Allingham. Novel Software Strategy for Glaucoma Diagnosis
Asymmetry Analysis of Retinal Thickness. _Arch Ophthalmol_ 129, 1205–1211 (2011). Article PubMed Google Scholar * Vizzeri, G. et al. Spectral domain-optical coherence tomography to detect
localized retinal nerve fiber layer defects in glaucomatous eyes. _Opt Express_ 17, 4004–4018 (2009). Article ADS CAS PubMed PubMed Central Google Scholar * Strouthidis, N., Yang, H.,
Fortune, B., Downs, J. & Burgoyne, C. Detection of the optic nerve head neural canal opening within three-dimensional histomorphometric and spectral domain optical coherence tomography
data sets. _Invest Ophthalmol Vis Sci_ 50, 214–223 (2009). Article PubMed Google Scholar * Burgoyne, C. F., Downs, J. C., Bellezza, A. J. & Hart, R. T. Three-dimensional
reconstruction of normal and early glaucoma monkey optic nerve head connective tissues. _Invest Ophthalmol Vis Sci_ 45, 4388–4399 (2004). Article PubMed Google Scholar * Downs, J. C. et
al. Three-dimensional histomorphometry of the normal and early glaucomatous monkey optic nerve head: neural canal and subarachnoid space architecture. _Invest Ophthalmol Vis Sci_ 48,
3195–3208 (2007). Article PubMed PubMed Central Google Scholar * Reis, A. S. et al. Laminar displacement and prelaminar tissue thickness change after glaucoma surgery imaged with optical
coherence tomography. _Invest Ophthalmol Vis Sci_ 53, 5819–5826 (2012). Article PubMed Google Scholar * Kim, T. W. et al. Imaging of the lamina cribrosa in glaucoma: perspectives of
pathogenesis and clinical applications. _Curr Eye Res_ 38, 903–909 (2013). Article PubMed PubMed Central Google Scholar * Agoumi, Y. et al. Laminar and prelaminar tissue displacement
during intraocular pressure elevation in glaucoma patients and healthy controls. _Ophthalmology_ 118, 52–59 (2011). Article PubMed Google Scholar * Na, J. H., Kook, M. S., Lee, Y., Yu, S.
J. & Choi, J. Detection of macular and circumpapillary structural loss in normal hemifield areas of glaucomatous eyes with localized visual field defects using spectral-domain optical
coherence tomography. _Graefes Arch Clin Exp Ophthalmol_ 250, 595–602 (2012). Article PubMed Google Scholar * Asrani, S., Zou, S., d’Anna, S., Vitale, S. & Zeimer, R. Noninvasive
mapping of the normal retinal thickness at the posterior pole. _Ophthalmology_ 106, 269–273 (1999). Article CAS PubMed Google Scholar * Asrani, S. et al. Correlation between retinal
thickness analysis, optic nerve and visual fields in glaucoma patients and suspects. _J Glaucoma_ 12, 119–128 (2003). Article PubMed Google Scholar * Wollstein, G., Ishikawa, H., Wang,
J., Beaton, S. A. & Schuman, J. S. Comparison of three optical coherence tomography scanning areas for detection of glaucomatous damage. _Am J Ophthalmol_ 139, 39–43 (2005). Article
PubMed Google Scholar * Johnson, C. A., Sample, P. A., Cioffi, G. A., Liebmann, J. R. & Weinreb, R. N. Structure and function evaluation (SAFE): I. Criteria for glaucomatous visual
field loss using standard automated perimetry (SAP) and short wavelength automated perimetry (SWAP). _Am J Ophthalmol_ 134, 177–185 (2002). Article PubMed Google Scholar * Bagga, H.,
Greenfield, D. S. & Knighton, R. W. Macular symmetry testing for glaucoma detection. _J Glaucoma_ 14, 358–363 (2005). Article PubMed Google Scholar * Moreno, P. A. et al.
Spectral-domain optical coherence tomography for early glaucoma assessment: analysis of macular ganglion cell complex versus peripapillary retinal nerve fiber layer. _Can J Ophthalmol_ 46,
543–547 (2011). Article PubMed Google Scholar * Chauhan, B. C. & Burgoyne, C. F. From clinical examination of the optic disc to clinical assessment of the optic nerve head: a paradigm
change. _Am J Ophthalmol_ 156, 218–227.e2 (2013). Article PubMed PubMed Central Google Scholar * Reis, A. S. et al. Influence of clinically invisible, but optical coherence tomography
detected, optic disc margin anatomy on neuroretinal rim evaluation. _Invest Ophthalmol Vis Sci_ 53, 1852–1860 (2012). Article PubMed PubMed Central Google Scholar * Fatehee, N., Yu, P.
K., Morgan, W. H., Cringle, S. J. & Yu, D. Y. Correlating morphometric parameters of the porcine optic nerve head in spectral domain optical coherence tomography with histological
sections. _Br J Ophthalmol_ 95, 585–589 (2011) Article PubMed Google Scholar * Hu, Z., Abramoff, M. D., Kwon, Y. H., Lee, K. & Garvin, M. K. Automated segmentation of neural canal
opening and optic cup in 3D spectral optical coherence tomography volumes of the optic nerve head. _Invest Ophthalmol Vis Sci_ 51, 5708–5717 (2010). Article PubMed PubMed Central Google
Scholar * Reis, A. S. et al. Optic disc margin anatomy in patients with glaucoma and normal controls with spectral domain optical coherence tomography. _Ophthalmology_ 119, 738–747 (2012).
Article PubMed PubMed Central Google Scholar * Strouthidis, N. G., Yang, H., Downs, J. C. & Burgoyne, C. F. Comparison of clinical and three-dimensional histomorphometric optic disc
margin anatomy. _Invest Ophthalmol Vis Sci_ 50, 2165–2174 (2009). Article PubMed PubMed Central Google Scholar * Quigley, H. A., Hohman, R. M., Addicks, E. M., Massof, R. W. & Green,
W. R. Morphologic changes in the lamina cribrosa correlated with neural loss in open-angle glaucoma. _Am J Ophthalmol_ 95, 673–691 (1983). Article CAS PubMed Google Scholar *
Strouthidis, N. G., Fortune, B., Yang, H., Sigal, I. A. & Burgoyne, C. F. Longitudinal change detected by spectral domain optical coherence tomography in the optic nerve head and
peripapillary retina in experimental glaucoma. _Invest Ophthalmol Vis Sci_ 52, 1206–1219 (2011). Article PubMed PubMed Central Google Scholar * Lee, K. M., Lee, E. J. & Kim, T. W.
Lamina cribrosa configuration in tilted optic discs with different tilt axes: a new hypothesis regarding optic disc tilt and torsion. _Invest Ophthalmol Vis Sci_ 56, 2958–2967 (2015) Article
PubMed Google Scholar * Mari, J. M., Strouthidis, N. G., Park, S. C. & Girard, M. J. Enhancement of lamina cribrosa visibility in optical coherence tomography images using adaptive
compensation. _Invest Ophthalmol Vis Sci_ 54, 2238–2247 (2013). Article PubMed Google Scholar * Lee, E. J. et al. Three-dimensional evaluation of the lamina cribrosa using spectral-domain
optical coherence tomography in glaucoma. _Invest Ophthalmol Vis Sci_ 53, 198–204 (2012). Article PubMed Google Scholar * Rebolleda, G. & Muñoz Negrete, F. J. Enhanced Depth Imaging-
optical coherence tomography technique and the lamina cribrosa in glaucoma. _Arch Soc Esp Oftalmol_ 89, 133–135 (2014). Article CAS PubMed Google Scholar * Wang, Ya li & Dong, yang
zeng. Clinical application of Optovue RTVue OCI’ and Heidelberg Retina Tomograph III in early diagnosis of glaucoma. _Chinese Journal of Experimental Ophthalmology_ 29, 249–253 (2011) Google
Scholar * Quigley, H. A. & Green, W. R. The histology of human glaucoma cupping and optic nerve damage: clinicopathologic correlation in 21 eyes. _Ophthalmology_ 86, 1803–1830 (1979).
Article CAS PubMed Google Scholar * Quigley, H. A., Addicks, E. M. & Green, W. R. Optic nerve damage in human glaucoma. III. Quantitative correlation of nerve fiber loss and visual
field defect in glaucoma, ischemic neuropathy, papilledema, and toxic neuropathy. _Arch Ophthalmol_ 100, 135–146 (1982). Article CAS PubMed Google Scholar Download references
ACKNOWLEDGEMENTS The manuscript has been edited by native English speakers (Nature Publishing Group Language Editing). In addition, Prof. Hongbo Liu, from the College of Public Health, China
Medical University, assisted with statistical analyses. This work was supported by 1. Liaoning Society Development Project (2012225012), and 2. National Natural Science Foundation of China
(81200656). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS *
Department of Ophthalmology, First Hospital of China Medical University, Shenyang, China Rui Hua & Xiaoli Ma * Department of Ophthalmology. The University of Hong Kong, Hong Kong, China
Rita Gangwani & Sarah McGhee * Ophthalmology and optometry center, First Hospital of China Medical University, Shenyang, China Lei Guo * Department of Ophthalmology and Visual Science,
Yale University School of Medicine, New Haven, CT 06511, USA Jun Li & Kai Yao Authors * Rui Hua View author publications You can also search for this author inPubMed Google Scholar *
Rita Gangwani View author publications You can also search for this author inPubMed Google Scholar * Lei Guo View author publications You can also search for this author inPubMed Google
Scholar * Sarah McGhee View author publications You can also search for this author inPubMed Google Scholar * Xiaoli Ma View author publications You can also search for this author inPubMed
Google Scholar * Jun Li View author publications You can also search for this author inPubMed Google Scholar * Kai Yao View author publications You can also search for this author inPubMed
Google Scholar CONTRIBUTIONS Study concept and design: R.H. and K.Y. Acquisition of data: R.H., X.M. and K.Y. Data analysis and interpretation: R.H., K.Y., L.G. and R.G. Drafting of the
manuscript: R.H., K.Y., L.G., R.G., S.M. and J.L. Critical revision of the manuscript for important intellectual content: R.H., K.Y., J.L. and L.G. All authors read and approved the final
manuscript. CORRESPONDING AUTHORS Correspondence to Lei Guo, Xiaoli Ma or Kai Yao. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. RIGHTS AND
PERMISSIONS This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s
Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the
license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Hua,
R., Gangwani, R., Guo, L. _et al._ Detection of preperimetric glaucoma using Bruch membrane opening, neural canal and posterior pole asymmetry analysis of optical coherence tomography. _Sci
Rep_ 6, 21743 (2016). https://doi.org/10.1038/srep21743 Download citation * Received: 07 April 2015 * Accepted: 26 January 2016 * Published: 17 February 2016 * DOI:
https://doi.org/10.1038/srep21743 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently
available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative