Molecular mechanisms underlying progesterone-enhanced breast cancer cell migration
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

Progesterone (P4) was demonstrated to inhibit migration in vascular smooth muscle cells (VSMCs), but to enhance migration in T47D breast cancer cells. To investigate the mechanism
responsible for this switch in P4 action, we examined the signaling pathway responsible for the P4-induced migration enhancement in breast cancer cell lines, T47D and MCF-7. Here, we
demonstrated that P4 activated the cSrc/AKT signaling pathway, subsequently inducing RSK1 activation, which in turn increased phosphorylation of p27 at T198 and formation of the
p27pT198-RhoA complex in the cytosol, thereby preventing RhoA degradation and eventually enhanced migration in T47D cells. These findings were confirmed in the P4-treated MCF-7. Comparing
the P4-induced molecular events in between breast cancer cells and VSMCs, we found that P4 increased p27 phosphorylation at T198 in breast cancer cells through RSK1 activation, while P4
increased p27 phosphorlation at Ser10 in VSMCs through KIS activation. P27pT198 formed the complex with RhoA and prevented RhoA degradation in T47D cells, whereas p-p27Ser10 formed the
complex with RhoA and caused RhoA degradation in VSMCs. The results of this study highlight the molecular mechanism underlying P4-enhanced breast cancer cell migration and suggest that RSK1
activation is responsible for the P4-induced migration enhancement in breast cancer cells.
In developed countries, breast cancer is the most commonly occurring female cancer. Endogenous sex hormones are thought to influence the risk of developing of breast cancer1. Studies of the
relationship between sex hormones and breast cancer in premenopausal women showed that the risk of breast cancer is positively associated with circulating concentrations of estrogens and
androgens2,3,4. Experimental and epidemiological studies also suggest that estrogen and P4 are intimately linked to mammary carcinogenesis. The clinical findings from the Women’s Health
Initiative and Million Women Study demonstrated that women taking progestin together with estrogen as part of hormone replacement experienced a greater breast cancer risk (larger tumor and
higher grade) as compared with taking estrogen alone5,6. However, some clinical trials have also shown that combined estrogen and P4 hormone replacement therapy is associated with a very
small increase in the risk of developing of breast cancer.
P4 is an ovarian steroid hormone. The central physiological roles of P4 in human reproduction include normal breast development during puberty, facilitation of implantation, maintenance of
pregnancy, regulation of the signaling required for sexual behavior in the brain7,8. The actions of P4 are primarily mediated by binding to its high-affinity receptors, P4 receptor (PR)-A
and/or PR-B isoforms. Co-treatment with P4 and estrogen are frequently prescribed for postmenopausal hormone replacement therapy. Estrogen has been indicated to be a potent breast mitogen
and inhibitors of the estrogen receptor, aromatases or estrogen-producing enzymes are effective first-line cancer therapies. Regarding the role of P4 in the development of breast cancer, P4
has been demonstrated to enhance proliferation9,10,11 and migration12 of breast cancer cells through extra-nuclear signaling pathways. Previously, it has been demonstrated that P4 drives
PR-A to interact with the G protein Gα13, whereas medroxyprogesterone acetate drives PR to interact with cSrc and to activate PI3K, leading to the activation of RhoA/ROCK-2 in breast cancer
cell lines12. However, the signaling pathway underlying P4-induced migration enhancement in breast cancer cells is still not fully elucidated.
In the present investigation, we used in vitro system to study how P4 affect the migration of T47D and MCF-7 breast cancer cell lines. These experimental findings reported below highlight
certain molecular mechanisms underlying P4-induced migration enhancement in breast cancer cells. Only when the molecular mechanism underlying P4-induced migration enhancement in breast
cancer cells is fully understood can we begin to design a strategy for treating the P4-enhanced breast cancer cell migration.
Previously, we demonstrated that inactivation of RhoA mediated by up-regulation of p27 is involved in the P4-induced migration inhibition in rat aortic smooth muscle cells (RASMCs)13. In the
present study, we investigated whether up-regulation of p27 is involved in the P4-induced migration enhancement in breast cancer cells. Initially, we used T47D breast cancer cell line to
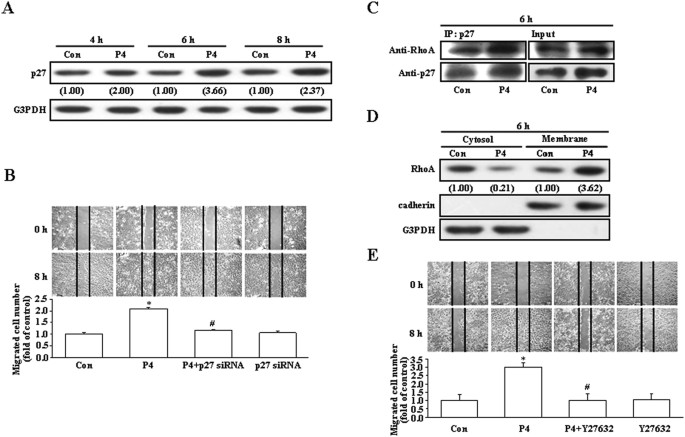
address this issue. As shown in Fig. 1A, treatment with P4 (50 nM) for 4–8 h increased the levels of p27 protein in T47D cells. However, pre-transfection with p27 siRNA significantly reduced
the P4-induced migration enhancement in T47D cells (Fig. 1B), suggesting that up-regulation of p27 contributed to the P4-induced migration enhancement in T47D cells. Since it has been
indicated that RhoA plays an important role in regulating cell motility, we next examined the involvement of RhoA activation in the P4-enhanced migration in T47D cells. Treatment with P4 for
6 h increased formation of the p27-RhoA complex (Fig. 1C) and membrane translocation of RhoA from the cytosol (Fig. 1D). Moreover, pre-treatment with Y27632 (5 μM), a ROCK inhibitor (a
kinase associated with RhoA for transducing RhoA signaling), prevented the P4-induced migration enhancement in T47D cells (Fig. 1E). These data suggest that RhoA activation is also involved
in the P4-enhanced migration in T47D cells.
Involvement of p27 and RhoA in the P4-induced enhanced migration in T47D cells.
(A) Treatment with P4 (50 nM) for 4–8 h increased the level of p27 protein in T47D cells. (B) Knock-down of p27 abolished the P4-induced migration enhancement in T47D cells. P4 increased
formation of the p27-RhoA complex (C) and membrane translocation of RhoA from the cytosol (D). (E) Pre-treatment with a ROCK inhibitor, Y27632 (5 μM), prevented the P4-induced migration
enhancement in T47D cells. For Western blot analyses, data are representative of 2 independent experiments with similar results. Values shown in parentheses represent the quantified results
adjusted with G3PDH (A) or with G3PDH and cadherin for the cytosol and the membrane, respectively (D) and expressed as ratio over its own control. cadherin and G3PDH were used as a
membranous and cytosolic protein marker, respectively, to confirm the purities of isolation and to verify equivalent sample loading. The gels have been run in the same experimental
conditions and the cropped blots were shown. The entire gel pictures of 1C were shown in the Supplemental Fig. 1. In (B,E), values represent the means±s.e.mean. (n = 3). *P