OnabotulinumtoxinA Urethral Sphincter Injection as Treatment for Non-neurogenic Voiding Dysfunction – A Randomized, Double-Blind, Placebo-Controlled Study
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

Non-neurogenic voiding dysfunction including dysfunctional voiding and detrusor underactivity caused by a spastic or non-relaxing external urethral sphincter can theoretically be treated by
injections of botulinum A toxin into the external urethral sphincter. This randomized, double-blind, placebo-controlled trial was designed to determine the clinical efficacy of
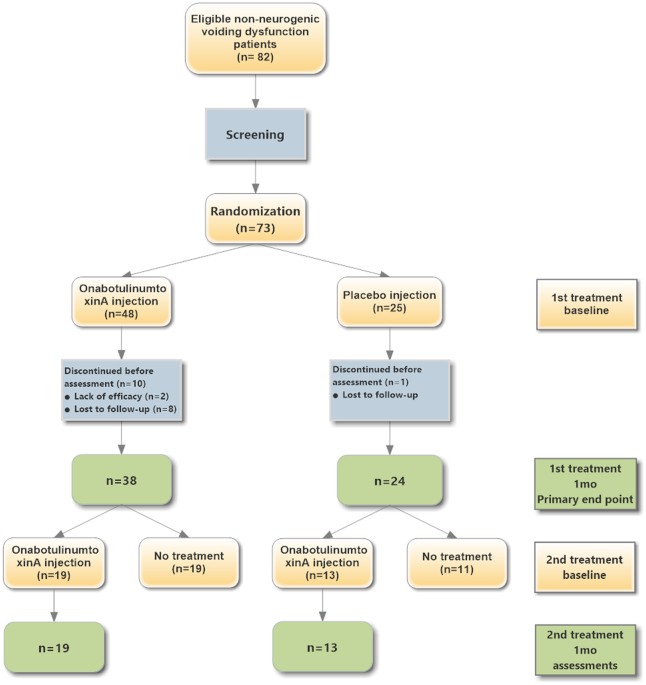
onabotulinumtoxinA urethral sphincter injections in patients with dysfunctional voiding or detrusor underactivity. Patients with medically refractory dysfunctional voiding (n = 31) or
detrusor underactivity (n = 31) were randomly allocated in a 2:1 ratio to receive either onabotulinumtoxinA (100 U) (n = 38) or placebo (normal saline) (n = 24). There were no significant
differences in subjective or objective parameters between patients who received onabotulinumtoxinA and those who received saline injection therapy, and the overall success rate was 43.5%
(reduction in Patient perception of Bladder Condition by ≥2: onabotulinumtoxinA 36.8% vs placebo 54.2%, p = 0.114). The results were similar between the dysfunctional voiding and detrusor
underactivity subgroups; however, a significant reduction in detrusor voiding pressure was only observed in dysfunctional voiding patients who received onabotulinumtoxinA. Repeat urethral
sphincter onabotulinumtoxinA injections offered greater therapeutic effects in both dysfunctional voiding and detrusor underactivity patients. For patients with non-neurogenic voiding
dysfunction, the success rate of onabotulinumtoxinA urethral sphincter injection was not superior to placebo.
Non-neurogenic voiding dysfunction presents therapeutic challenges to urologists because of the lack of consensus regarding diagnosis and definition as well as its broad range of causes,
including bladder outlet obstruction, dysfunctional voiding, detrusor underactivity and detrusor overactivity with impaired detrusor contractility1,2,3,4. A spastic or non-relaxing external
urethral sphincter is thought to be a possible cause of dysfunctional voiding and detrusor underactivity, resulting in voiding symptoms, slow or fractionated urinary flow, large post-void
residual urine, and sometimes deterioration of upper urinary tract function.
Botulinum toxin A, a potent neurotoxin, can inhibit the release of neurotransmitters from efferent nerve terminals at neuromuscular junctions, thereby paralyzing muscle5. Botulinum toxin A
injection into the urethral; sphincter is now widely applied as treatment for various types of lower urinary tract diseases, including neurogenic and non-neurogenic voiding dysfunction6. In
1988, Dykstra et al. reported the first application of onabotulinumtoxinA injection into external urethral sphincter to treat detrusor-sphincter dyssynergia in patients with spinal cord
injuries7. In 1997, Steinhardt et al. were the first to report that injections of onabotulinumtoxinA into the urethral sphincter of a neurologically normal child with refractory
dysfunctional voiding resulted in the resolution of urinary tract infections and incontinence episodes8. Since then, urethral sphincter onabotulinumtoxinA injection has been used to treat
various types of neurogenic or non-neurogenic voiding dysfunction, including chronic urinary retention9, detrusor underactivity10,11, poor relaxation of urethral sphincter12, and lower
urinary tract symptoms in men with small prostates13.
For detrusor sphincter dyssynergia, urethral sphincter injection with onabotulinumtoxinA 100 U was reported to achieve an overall satisfactory result of 60.6% with significant improvement in
the reduction of voiding detrusor pressure and post-void residual urine volume, and an increase in maximal urinary flow rate14. In 10 pediatric patients with dysfunctional voiding, urethral
sphincter injection with onabotulinumtoxinA 50–100 U resulted in self voiding without catheterization, increased maximum flow rate values and lower post-void residual volume in 90% of
patients15. Franco et al. reported that increasing the onabotulinumtoxinA dose to 200–300 U resulted in increased efficacy without increasing the morbidity rate16. Liao et al. found that
urethral sphincter injection with 50–100 U onabotulinumtoxinA resulted in an overall success rate of 86.7% in adults with dysfunctional voiding and a success rate of 95.7% in patients with
poor relaxation of the urethral sphincter12. By paralyzing the urethral sphincter and reducing urethral resistance, onabotulinumtoxinA injection therapy facilitates bladder emptying,
improves subjective symptoms and life quality, and reduces the need for catheterization10,11. In addition, Kuo et al. reported that detrusor contractility recovered in approximately 50% of
patients with detrusor underactivity who received urethral sphincter onabotulinumtoxinA injections, indicating that onabotulinumtoxinA has neuromodulation effects in the lower urinary
tract17. Urethral sphincter injection with onabotulinumtoxinA was widely applied for many lower urinary tract diseases.
Although urethral sphincter onabotulinumtoxinA injection has been shown to be an effective therapy for patients with voiding dysfunction, its application for non-neurogenic voiding
dysfunctions, including dysfunctional voiding and detrusor underactivity, is still off-label. Most studies on its effects have been retrospective and lacked a control arm. In addition,
onabotulinumtoxinA doses and the injection techniques varied widely among different study groups. Therefore, this prospective, randomized, double-blind, placebo-controlled trial was designed
to demonstrate the actual therapeutic efficacy of urethral sphincter onabotulinumtoxinA injections for the treatment of non-neurogenic voiding dysfunctions, including dysfunctional voiding
and detrusor underactivity.
Participants in this prospective, randomized, double-blind, placebo-controlled clinical trial comprised patients with a three-month history of medically refractory non-neurogenic voiding
dysfunction, namely dysfunctional voiding and detrusor underactivity. The diagnoses of dysfunctional voiding and detrusor underactivity were established based on the results of
video-urodynamic study, which were performed according to the recommendations of International Continence Society18. Dysfunctional voiding was diagnosed in patients who presented with an
open bladder neck but narrow membranous urethra (a spinning top appearance) on real-time fluoroscopy, a poorly relaxed urethral sphincter on electromyography, and a normal-to-high voiding
pressure with a low and/or intermittent urinary flow during voiding. Detrusor underactivity was diagnosed in patients with low voiding pressure and low flow rate, a post-vid residual volume
>300 mL, and a low voiding efficiency (