Decreased nox2 expression in the brain of patients with bipolar disorder: association with valproic acid prescription and substance abuse
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Neuroinflammation and increased oxidative stress are believed to contribute to the development of psychiatric diseases. Animal studies have implicated NADPH oxidases (NOX) as
relevant sources of reactive oxygen species in the brain. We have analyzed the expression of NOX isoforms in post-mortem brain samples from patients with psychiatric disorders
(schizophrenia, bipolar disorder) and non-psychiatric subjects. Two collections from the Stanley Medical Research Institute were studied: the Array Collection (RNA, 35 individuals per
group), and a neuropathology consortium collection (paraffin-embedded sections, 15 individuals per group). Quantitative PCR analysis revealed expression of NOX2 and NOX4 in prefrontal
cortex. No impact of psychiatric disease on NOX4 levels was detected. Remarkably, the expression of NOX2 was specifically decreased in prefrontal and cingulate cortices of bipolar patients,
as compared with controls and schizophrenic patients. NOX2 expression was not statistically associated with demographic parameters and post-mortem interval, but correlated with brain pH.
Immunostaining demonstrated that NOX2 was predominantly expressed in microglia, which was corroborated by a decrease in the microglial markers CD68 and CD11b in the cingulate cortex of
bipolar disorder patients. The analysis of potentially confounding parameters showed association of valproic acid prescription and heavy substance abuse with lower levels of NOX2. Taken
together, we did not observe changes of NOX2 in schizophrenic patients, but a marked decrease of microglial markers and NOX2 in the brain of bipolar patients. This might be an underlying
feature of bipolar disorder and/or a consequence of valproic acid treatment and substance abuse. SIMILAR CONTENT BEING VIEWED BY OTHERS EVALUATION OF NDEL1 OLIGOPEPTIDASE ACTIVITY IN BLOOD
AND BRAIN IN AN ANIMAL MODEL OF SCHIZOPHRENIA: EFFECTS OF PSYCHOSTIMULANTS AND ANTIPSYCHOTICS Article Open access 28 October 2020 DISTINCT PROTEOMIC PROFILES IN PREFRONTAL SUBAREAS OF
ELDERLY MAJOR DEPRESSIVE DISORDER AND BIPOLAR DISORDER PATIENTS Article Open access 11 July 2022 DIFFERENTIAL EXPRESSION OF GENE CO-EXPRESSION NETWORKS RELATED TO THE MTOR SIGNALING PATHWAY
IN BIPOLAR DISORDER Article Open access 04 May 2022 INTRODUCTION Psychiatric diseases, including depression, bipolar disorder (BD) and schizophrenia (SZ), are a major challenge to public
health. Mechanisms underlying their pathogenesis are still poorly understood, but evidence points toward a combination of genetic and environmental factors. Patients with mental disorders
are given symptomatic treatments, such as antipsychotics (for example, clozapine) for schizophrenics, mood stabilizers (lithium, valproic acid (VPA)) for bipolar patients. Many of these
drugs have multiple modes of action, including anti-oxidative1 and anti-inflammatory activities.2 Although BD and SZ are distinct diagnoses, neuropathological differences and biochemical
quantitative biomarkers distinguishing the two disorders are virtually non-existing. SZ and BD symptoms are overlapping and patients are often given similar treatments. At the
neuropathological level, most evidence argues for a contribution of neuroinflammation, dysfunction in glutamate neurotransmission, altered microglial function and oxidative stress. Changes
in reactive oxygen species (ROS) generation are commonly observed in neuronal disorders and psychiatric diseases make no exception. Oxidative modifications are documented in SZ or BD
patients. This includes higher levels of 4-hydroxynonenal (peroxidized lipids),3 oxidized guanine in post-mortem brain samples4, 5 as well as increased thiobarbituric acid reactive
substances (byproducts of lipid peroxidation)6 and decreased levels of intracellular antioxidants in the blood.7 Also, plasma levels of the oxidized form of cysteine were found to be
elevated in schizophrenic8, 9 and bipolar patients. Similarly, several animal models of psychosis recapitulate observations made in humans and present increased oxidation in both central
nervous system (CNS) parenchyma and the blood (reviewed in ref. 10). It therefore has been speculated, that the increased oxidative stress in the CNS is due to an increased ROS generation
(see below) and/or to a decrease in antioxidant capacity and in enzymes mediating ROS degradation. For example, genetic associations between polymorphisms in enzymes involved in the
glutathione synthesis and SZ have been described,11, 12 which might account for low glutathione levels found in SZ.13 Sources of ROS in the CNS are multiple, and their relative contribution
to oxidative stress is a subject of intense investigations. NADPH oxidases (NOX), in particular, the phagocyte NADPH oxidase NOX2 might be important.14 A role for NOX2 in psychiatric
disorders was first proposed in a study from Behrens and collaborators, which use a drug-induced model of psychosis (administration of the NMDA antagonist ketamine to mice). The authors
proposed that increased ROS generation by neuronal NOX2 is causative in psychogenesis and induced the loss of parvalbumin-positive neurons, a key pathological hallmark of the schizophrenic
brain. Interestingly, ketamine, has received much attention as a promising drug for the treatment of major depression,15 raising the possibility that some fundamental differences in redox
systems regulate SZ and depressive psychiatric disorders. Although animal models of psychosis may shed light on several redox-dependent processes, translation of these findings to human is
required. Clinical trials using the reducing agent _N_-acetylcysteine have shown encouraging results on some specific symptoms of SZ and BD (reviewed in ref. 16). However, redox mechanisms
in the human brain and their relationship to psychiatric diseases remain insufficiently understood. Clinical studies using material collected from psychiatric patients should help filling
the gap between human and mouse studies, and address the role of NOX in psychiatric diseases. In this study, we investigated the expression of NOX in the brain of patients diagnosed with BD
or SZ. MATERIALS AND METHODS POST-MORTEM BRAIN SAMPLES For the studies of gene expression, we used mRNA from cingulate cortex and prefrontal cortex (PFC) from the Array Collection provided
by Stanley Medical Research Institute. Selection of samples, informed consent and criteria of exclusion are documented in http://www.stanleyresearch.org/brain-research. The collection
consists of 105 brains from patients diagnosed with SZ, BD and unaffected controls (35 cases in each group). Demographic information is provided in Supplementary Table S1. For
immunostaining, we used fixed paraffin 10 μm thick sections from anterior cingulate cortex from the Neuropathology Consortium collection provided by Stanley Medical Research Institute. This
collection consists of 60 brains from patients diagnosed with SZ, BD, depression and unaffected controls (15 each). In this study, we focused on three groups (SZ, bipolar and controls)
matched by age, sex, race, post-mortem interval, pH and side of brain. Diagnosis and socio-demographic distribution of patients considered in this study are shown in Supplementary Table S2.
The study was approved by the Stanley Medical Research Institute. REAL-TIME QUANTITATIVE POLYMERASE CHAIN REACTION Total RNA (500 ng) was used for cDNA synthesis using the superscript II kit
according to the manufacturer’s instructions (Invitrogen, Basel, Switzerland). Real-time PCR was performed using SYBR green assay on a 7900HT SDS systems from ABI at the Genomics Platform,
National Center of Competence in Research Frontiers in Genetics, Geneva. The efficiency of each primer was verified with serial dilutions of cDNA. Relative expression levels were calculated
by normalization to geometric mean of the three house-keeping genes (GAPDH, β-2-microglobulin and EEF1A1) as described previously.17 The highest normalized relative quantity was arbitrarily
designated as a value of 1. Fold-changes were calculated from the quotient of means of these normalized quantities and reported as ±s.e.m. Sequences of all primers used in this study are
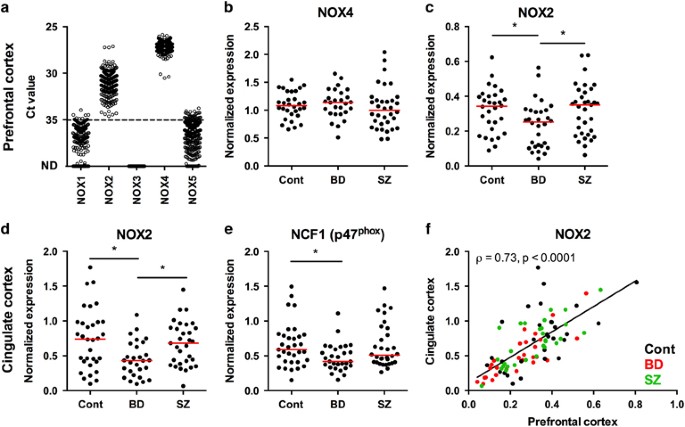
provided in Supplementary Table S3. Threshold cycle (Ct) values for NOX isoforms corresponding to the intersection between an amplification curve and a threshold line set at 0.20 by the
software are provided in Figure 1a. Specificity of NOX2 and NOX4 primers was verified by subcloning the generated amplicons using the _TOPO_ TA Cloning Kit (Thermo Fischer Scientific,
Reinach, Switzerland) followed by sequencing at Microsynth (Balgach, Switzerland). IMMUNOHISTOCHEMISTRY AND IMAGING Brain sections were deparaffinized through graded alcohols, subjected to
heat-induced epitope retrieval for 15 min in 0.01 mol l−1 citrate buffer (pH 6), and incubated overnight at 4 °C in PBS-0.3% Triton-X100 with the monoclonal anti-human NOX2 antibody Mo4818
(1:250 LSBio LS-C85347, Seattle, WA, USA). Sections were then incubated for 1 h at room temperature with specific biotinylated secondary antibody (1:100, Vector Laboratories, Peterborough,
UK) and, after several washes in PBS, for 1 h in horseradish peroxidase-avidin/biotin complex solution (1:100, Vector Laboratories) and detected with 3,3-diaminobenzidinetetrahydrochloride
hydrate (DAB, Sigma-Aldrich, St Louis, MO, USA) and H2O2. For PFC, the images were acquired using a Digital Image Hub (Leica Biosystems, Wetzlar, Germany) and number of brown/white objects
was quantified using in-house—developed software analyzed as previously described.19 STATISTICAL ANALYSIS Spearman’s rank correlations were calculated for the relationship between NOX2
expression and all available clinical data to determine whether any other factors were contributing to the variation seen in the expression. The difference between two groups was studied
with the Mann–Whitney _U_ test; groups of more than two were studied using the nonparametric Kruskal–Wallis method with the application of a Dunn’s correction for multiple comparisons. Data
is presented as median on all figures. To examine for effects of continuous confounding measures, we performed Spearman’s correlations on measures that emerged as significant with the
nonparametric analyses of variance. There were nine continuous confounding measures available for this analysis (post-mortem interval, age, estimated lifetime exposure to neuroleptics, age
of illness onset, illness duration, brain pH, brain weight, and freezer or fixation storage time). There were also three non-continuous confounding measures (hemisphere, gender, and any
current or past history of substance abuse) available for analysis. These were used as grouping measures for Mann–Whitney _U_ tests for any abnormal measure. The level of significance was
defined as _P_<0.05. All statistical analyses were performed using Sigma Stat (version 3.0, San Jose, CA, USA), GraphPad Prism (version 4.0, La Jolla, CA, USA) and SPSS (version 10,
Armonk, NY, USA). BLINDNESS OF THE STUDY Researchers performing qPCR of NOX isoforms and NOX2 immunostaining analysis were blinded to patient diagnosis as well as to socio-demographic
distributions. Patients were initially identified by a code. Blindness of the study was broken after returning results to the Stanley Medical Research Institute. Further qPCR using the NOX2
subunit p47phox (NCF1) and the microglia markers CD11b, Iba1 and CD68 were subsequently performed. RESULTS EXPRESSION OF NOX MRNA IN PREFRONTAL AND CINGULATE CORTICES OF PSYCHIATRIC PATIENTS
To evaluate the expression of NOX NADPH oxidases in brain regions associated with psychiatric disorders we obtained RNA samples from PFC and cingulate cortex from the Stanley Array
Collection. The expression of mRNA for five NOX isoforms (NOX1–NOX5) in PFC was measured by qPCR. We did not analyze the expression of two other members of NOX family: DUOX1 and DUOX2 as
previous qPCR and RNAseq data suggest that they are not expressed in the brain tissue.20, 21 We found medium to high levels of NOX2 and NOX4 mRNA, and very low levels of NOX1 and NOX5,
whereas NOX3 was below the detection level (Figure 1a). We confirmed the identity of NOX2 and NOX4 by sequencing the qPCR products (data not shown). The Stanley Array Collection is a
collection of mRNA samples from patients with BD, SZ and healthy individuals without psychiatric diagnosis. We analyzed the expression of NOX2 and NOX4 in disease groups. No changes of NOX4
mRNA levels were found in the three analyzed groups (Figure 1b). In contrast to what has been suggested from mouse studies,22, 23 we did not observe significant changes of NOX2 expression in
the SZ group. However, we found a significant decrease of NOX2 mRNA levels in bipolar patients (_n_=28; median=0.4316; interquartile range (IQR) =0.4271), which distinguished this group
both from controls (_n_=32; median=0.7413; IQR=0.5687) and from schizophrenic patients (_n_=31; median=0.6802; IQR=0.5181) (Figure 1c). Interestingly, this decrease was not limited only to
PFC. Levels of NOX2 and its subunit p47phox were also decreased in cingulate cortex (Figures 1e and f). Overall, a strong correlation was found for NOX2 mRNA expression between the two brain
regions (Figure 1d). ASSOCIATION OF NOX EXPRESSION WITH DEMOGRAPHIC, SOCIAL AND TECHNICAL FACTORS The analysis of demographic factors (age, sex, brain weight and hemisphere), technical
factors related to sample preparation (post-mortem interval, refrigerator period (from estimated time of death to refrigeration of body) and of disease-related factors (age of onset, time in
the hospital) showed no statistically significant correlation neither with NOX2 expression in PFC and cingulate cortex (Supplementary Figure 1) nor with NOX4 expression in PFC
(Supplementary Figure 2). The expression of NOX2 significantly correlated with brain pH (Supplementary Figures 1i and s). It was previously shown that the brain pH in bipolar patients from
the Stanley Array Collection was lower than in the control group.24 This observation can have multiple interpretations including post-mortem sample preservation or changes of brain pH
inherent to disease. In our data set, the brain pH was also lower in bipolar patient as well as in SZ patients as compared with the controls (data not shown). It has been suggested that
decreased pH is associated with lower RNA quality.24 However, it is unlikely that the decrease in NOX2 expression in bipolar patients is due to the lower pH for the following reasons: (i)
the expression of NOX4 was not affected (Supplementary Figures 2i and j); (ii) no association between NOX2 and post-mortem interval (Supplementary Figures 1e and m) nor with refrigerator
interval (Supplementary Figures 1f and n) were found; (iii) NOX2 mRNA levels were not decreased in SZ patients and (iv) NOX2 levels were significantly lower even after elimination from the
analysis of samples with pH lower than 6.4 in PFC (Supplementary Figures 1j and t). Furthermore, no statistical impact of VPA prescription or substance abuse on pH was identified (data not
shown). Out of the social factors (smoking, alcohol consumption and substance abuse) and of the disease-related factors (psychotic features, suicide status and duration of illness) only
substance abuse was significantly associated with decreased expression of NOX2 both in PFC (Figure 2b: no/low users; _n_=58; median=0.739; IQR=0.5534 vs users; _n_=32; median=04372;
IQR=0.3971) and in cingulate cortex (Figure 2h: no/low users; _n_=58; median=0.3429; IQR=0.2239 vs users; _n_=32; median=0.2485; IQR=0.2024). LOCALIZATION OF NOX2 IN MICROGLIA AND
CORRELATION WITH MICROGLIAL MARKERS To address the question of NOX2 localization in the brain of psychiatric patients, we stained paraffin-embedded sections of PFC with a monoclonal
anti-human NOX2 antibody. The Mo48 antibody is currently the only validated antibody specific for human NOX2 in immunohistochemistry applications. Its epitope is known25 and Mo48 is
routinely used (also with the 7D5 monoclonal antibody) to characterize patients with NOX2 loss of function.26 Immunohistochemical analysis detected NOX2 staining mainly in microglia (Figures
3a–f). We quantified microglia separately from gray and white matter because of intrinsic differences in microglial density in these regions.27 No difference in object count was detected
between different disease groups in gray (Figure 3g) and in white matter (Figure 3h) in spite of a trend for a decrease in the white matter of the PFC. Recently RNASeq analysis of specific
cell populations of the mouse and human CNS (neurons, astrocytes, oligodendrocytes, microglia/macrophages and endothelial cells) has been made publicly available.20 We therefore analyzed the
expression of NOX2 (aka _CYBB_) in these samples (Figure 3i). By far, the largest expression of NOX2 was found in microglia/macrophages. There was also some degree of NOX2 expression in the
oligodendrocyte fraction; however, according to the authors of the publication, there was an ~5% contamination of microglia/macrophages population within the oligodendrocyte fraction.
Finally, the amount of NOX2 in astrocytes, endothelial cells and neurons was negligible. This corroborates our immunohistochemical data suggesting that the large majority of NOX2 in the
studied human brain samples are localized to microglia/macrophages. If NOX2 is indeed located in microglia, one would predict a correlation between the expression of NOX2 and microglia
markers. We therefore performed additional qPCR with mRNA samples from the cingulate cortex to evaluate the expression of microglial markers. We found a significant correlation between the
expression of NOX2 and microglial markers: CD11b, Spearman _ρ_=0.87, _P_<0.0001 (Figure 3j); CD68, Spearman _ρ_=0.85, _P_<0.0001 (Supplementary Figure 3a); Iba1, Spearman _ρ_=0.34,
_P_<0.0008 (Supplementary Figure 3b). The analysis of microglial markers in patient groups showed that the expression of CD11b (Figure 3k) and CD68 (Figure 3l) was significantly decreased
in BD group as compared with control. There were no significant differences for IBA-1 (Figure 3m). This is in line with the weaker correlation between NOX2 and Iba1 mRNA expression
(_ρ_=0.34; see above and Supplementary Figure 3b). This difference might be due to different activation stages of microglia in these samples and thereby may illustrate the relative
specificity of microglial markers in identifying surveillant versus activated microglia. ASSOCIATION OF NOX EXPRESSION WITH PRESCRIBED MEDICATIONS AND SUBSTANCE ABUSE Almost all patients in
our study were prescribed multiple medications including antipsychotics, mood stabilizers and antidepressants. Most patients, have a history of smoking, alcohol consumption and substance
abuse, in particular, those with BDs. These substances might impact microglia homeostasis.28 By investigating the association of NOX expression levels with the documented prescribed
medications, we found that the decrease of NOX2 levels in PFC and in cingulate cortex of BD patients was significantly associated with the prescription of VPA (Figures 4a and c). Such
association was not found for NOX4 (PFC, Figure 4b). There was also a decrease in microglia markers associated with VPA prescription (CD11b (Figure 4d), CD68 (Figure 4e), but not with Iba1
(Figure 4g). The prescription of another frequently used mood stabilizer, lithium, was not associated with the decrease in NOX2 levels nor with microglial markers. Note that no information
is available regarding the fact that patients would actually use VPA. No association between VPA prescription and a particular BD subtype was observed (data not shown). As VPA is often
prescribed to both BD and SZ patients, we compared these two patient groups. NOX2 expression was lower in BD patients receiving VPA than in schizophrenic patients to whom the drug has been
prescribed (Figures 5a and c). There was no statistically significant difference between schizophrenic patients receiving or not receiving VPA (albeit there was a trend). These data were
corroborated by expression levels of the activated microglia marker CD11b (Figure 5d). In contrast, VPA has no impact on NOX4 levels in our analysis (Figure 5b). We also performed a similar
analysis among subpopulations of patients with documented substance abuse. NOX2 expression was significantly decreased only between BD patients with substance use and abstinent SZ in PFC and
cingulate cortex, although there was a trend for a decrease of NOX2 and CD11b in BD users. No difference was detected for NOX4. The groups were probably too small to generate more
significant data. In addition, the substance use is a vague terminology and most likely represents very diverse drug-related behaviors and various types of recreational drug with
fundamentally different mode of action. DISCUSSION In this study we demonstrate—as opposed to what had been predicted based on animal experimentation—that there is no increase of NOX2
expression in schizophrenic patients. Unexpectedly, however, we found that NOX2 expression was decreased in patients with BD. This decrease was essentially seen in patients that were either
prescribed VPA or in patients with a history of substance abuse. NOX2 expression was mostly found in microglia suggesting a decreased activity of brain inflammatory cells in a subset of
patients with BD. We evaluated the expression of the ROS-generating NADPH oxidases NOX1-5 in human brain samples from the Stanley Collection (schizophrenic, BDs and control). The overall
pattern of expression was as follows: we found high mRNA expression levels of the NOX2 and NOX4, whereas NOX1 and NOX5 were at the limit of detection and NOX3 below detection levels.
Although the expression of NOX2 in the human brain has been convincingly documented before, the question of which cell type within the CNS expresses NOX2 is still a matter of debate.
Expression in microglia has been previously shown,19, 21 but NOX2 expression has been suggested in mature neurons,29 as well as adult neural stem cells.30 The interpretation of these—at
least in part contradictory—results is complex: (i) in many instances, antibodies with a poorly documented specificity have been used (discussed in ref. 14); (ii) the cell type that
expresses NOX2 might depend on the brain region and developmental state; and (iii) disease-specific expression of NOX2 might also exist. Our findings in prefrontal and cingulate cortices of
psychiatric patients and control individuals argue in favor of a specific expression of NOX2 in microglia: (i) brain immunostaining with the specific and fully validated NOX2 antibody
mo48[ref. 18] detected cells with an obvious microglial morphology, and (ii) NOX2 expression correlated with macrophage/microglial markers CD11b, CD68 and Iba1. Along these lines, data
extracted from an RNAseq study31 indicates strong microglial enrichment of NOX2 and its subunits p47phox, p67phox and p40phox, whereas NOX2 was virtually absent in neurons. Data from mouse
experiments suggest that NOX2 is also expressed in adult neural stem cells of the subventricular zone and the dentate gyrus.30, 32 However, this has not been investigated in humans, and, in
addition, neural stem cells are most likely absent in the regions (cingulate and prefrontal cortices) investigated in our study. We did not address the cellular CNS localization of NOX4 by
immunohistochemistry, but recent RNAseq data indicate a strong NOX4 enrichment in human brain endothelial cells,33 whereas microarray data indicate NOX4 expression in human pericytes.34 A
major goal of our study was to understand whether NOX2 expression was altered in psychiatric disease. SZ and BD are distinct and yet overlapping clinical entities. The etiologies of the two
psychiatric diseases remain poorly understood. Several lines of evidence argue for a role of inflammation35 and oxidative dysregulation.36 Oxidative modifications in the brain of
schizophrenic patients have been widely reported10 making NOX2 an attractive candidate to be involved in the disease process. On the basis of animal models of psychosis,37 a role of
increased NOX2 activity and expression in the pathophysiology of schizophrenia has been suggested. Other NOX isoforms have hardly been studied in this context. To our knowledge, our study
represents the first systematic analysis of NOX enzymes in samples from patients with psychiatric disease. The NOX1, NOX3 and NOX5 isoforms showed very low expression levels, and for this
reason we did not perform in-depth analysis. NOX4 expression levels were high, but we did not observe any changes between patient groups, socio-demographic factors, and technical parameters.
However, marked variations in NOX2 expression levels were detected. Our initial working hypothesis, that there might be an increased level of NOX2 in schizophrenic patients was not
confirmed. Indeed, NOX2 levels were virtually identical in schizophrenic patients and in control. Unexpectedly, however, we found that BD patients—as compared to controls and schizophrenic
patients—had significantly lower levels of NOX2 in both brain regions tested. The most widely used microglia marker Iba1, is not changed in BD patients. However, the activation-dependent
microglia markers CD11b and CD68 follow a similar pattern as NOX2, suggesting that microglia activation rather than microglia number is affected. This robust decrease of NOX2 and other
microglial activation markers in BD post-mortem material is a novel and unexpected finding. Indeed, most publications support a role of exaggerated microglia activity and increased
pro-inflammatory cytokines in psychiatric diseases. Our study challenges this theory. Importantly, our results indicate that there are differences in microglial response in BD and SZ. Our
results raise the possibility that decreased NOX2 expression is linked to BD. At this point, however, it is difficult to distinguish whether this is an intrinsic feature of the disease or
whether this is due to external factors. At the first glance, our data appears to favor the latter explanation: decreased NOX2 expression is limited to a subgroup of BD patients, namely
patients that have been prescribed VPA, and patients with a history of heavy substance abuse. Thus, one working hypothesis might be that VPA and recreational drugs suppress NOX2 expression.
VPA is drug with a complex mode of actions, including inhibitions of histone deacetylases.38 It is used since the 1960s for the treatment of several CNS disorders, including epilepsy and
BDs. High concentrations of VPA have been shown to be inhibitory to human microglia39 or even toxic to mouse microglia40 and/or rat peripheral leukocytes.41 Thus, the decreased NOX2
expression and microglial activation could be due to VPA treatment. Although this possibility is real, we are not aware of a common biochemical pathway that links the use of VPA and
recreational drugs. No information on the compliance towards VPA and the type of substances abused by the patients is available for the Stanley Collection. Also, a decrease in NOX2 is
observed only in BD, but not in SZ patients with VPA prescription. For this reason, an alternative explanation should also be considered. It is conceivable that patients that are prescribed
VPA and patients prone to substance abuse belong to a pathophysiologically distinct subgroup of BD patients. Indeed, VPA is often prescribed to BD patients that are not responsive or
intolerant to lithium.42 Thus, a more generalized microglia pathology might contribute to the pathophysiology of this subgroup of BD patients. Primary alterations in microglia function have
been implicated in autism43 and obsessive compulsive behavior.44 Assuming that decreased microglia activation in BD is not a drug-induced epiphenomenon, how could it contribute to disease
progression? Microglia has a key role in brain homeostasis by regulating neurogenesis both during development and in adults by removing superfluous synapses (synaptic pruning).45, 46 Thus,
the decreased microglia activity in BD patients might alter synaptic pruning. Alternatively, one might consider a paracrine pathway where NOX2-derived ROS regulate redox-sensitive
transcription factors and signaling in neural cells. Altogether, our results demonstrate low microglial NOX2 in a subgroup of patients with BD, whereas NOX2 expression and microglial
activation were unchanged in schizophrenia. The decrease in NOX2 expression might either be secondary to VPA and recreational drugs, or represent a pathophysiologically relevant feature of a
subgroup of BD patients. In the future, it will be important to develop techniques of _in vivo_ redox imaging in the human brain, which will allow distinguishing between these
possibilities. REFERENCES * Data-Franco J, Singh A, Popovic D, Ashton M, Berk M, Vieta E _et al_. Beyond the therapeutic shackles of the monoamines: new mechanisms in bipolar disorder
biology. _Prog Neuro-psychopharmacol Biol Psychiatry_ 2017; 72: 73–86. Article CAS Google Scholar * Kato TA, Monji A, Mizoguchi Y, Hashioka S, Horikawa H, Seki Y _et al_.
Anti-Inflammatory properties of antipsychotics via microglia modulations: are antipsychotics a 'fire extinguisher' in the brain of schizophrenia? _Mini Rev Med Chem_ 2011; 11:
565–574. Article CAS Google Scholar * Romano A, Serviddio G, Calcagnini S, Villani R, Giudetti AM, Cassano T _et al_. Linking lipid peroxidation and neuropsychiatric disorders: focus on
4-hydroxy-2-nonenal. _Free Radic Biol Med_ 2017. * Che Y, Wang JF, Shao L, Young T . Oxidative damage to RNA but not DNA in the hippocampus of patients with major mental illness. _J
Psychiatry Neurosci_ 2010; 35: 296–302. Article Google Scholar * Andreazza AC, Wang JF, Salmasi F, Shao L, Young LT . Specific subcellular changes in oxidative stress in prefrontal cortex
from patients with bipolar disorder. _J Neurochem_ 2013; 127: 552–561. Article CAS Google Scholar * Andreazza AC, Kauer-Sant'anna M, Frey BN, Bond DJ, Kapczinski F, Young LT _et al_.
Oxidative stress markers in bipolar disorder: a meta-analysis. _J Affect Disord_ 2008; 111: 135–144. Article CAS Google Scholar * Raffa M, Barhoumi S, Atig F, Fendri C, Kerkeni A, Mechri
A . Reduced antioxidant defense systems in schizophrenia and bipolar I disorder. _Prog Neuro-psychopharmacol Biol Psychiatry_ 2012; 39: 371–375. Article CAS Google Scholar * Gysin R,
Kraftsik R, Boulat O, Bovet P, Conus P, Comte-Krieger E _et al_. Genetic dysregulation of glutathione synthesis predicts alteration of plasma thiol redox status in schizophrenia. _Antioxid
Redox Signal_ 2011; 15: 2003–2010. Article CAS Google Scholar * Gawryluk JW, Wang JF, Andreazza AC, Shao L, Young LT . Decreased levels of glutathione, the major brain antioxidant, in
post-mortem prefrontal cortex from patients with psychiatric disorders. _Int J Neuropsychopharmacol_ 2011; 14: 123–130. Article CAS Google Scholar * Kulak A, Steullet P, Cabungcal JH,
Werge T, Ingason A, Cuenod M _et al_. Redox dysregulation in the pathophysiology of schizophrenia and bipolar disorder: insights from animal models. _Antioxid Redox Signal_ 2013; 18:
1428–1443. Article CAS Google Scholar * Gysin R, Kraftsik R, Sandell J, Bovet P, Chappuis C, Conus P _et al_. Impaired glutathione synthesis in schizophrenia: convergent genetic and
functional evidence. _Proc Natl Acad Sci USA_ 2007; 104: 16621–16626. Article CAS Google Scholar * Tosic M, Ott J, Barral S, Bovet P, Deppen P, Gheorghita F _et al_. Schizophrenia and
oxidative stress: glutamate cysteine ligase modifier as a susceptibility gene. _Am J Hum Genet_ 2006; 79: 586–592. Article CAS Google Scholar * Do KQ, Trabesinger AH, Kirsten-Kruger M,
Lauer CJ, Dydak U, Hell D _et al_. Schizophrenia: glutathione deficit in cerebrospinal fluid and prefrontal cortex _in vivo_. _Eur J Neurosci_ 2000; 12: 3721–3728. Article CAS Google
Scholar * Nayernia Z, Jaquet V, Krause KH . New insights on NOX enzymes in the central nervous system. _Antioxid Redox Signal_ 2014; 20: 2815–2837. Article CAS Google Scholar * Scheuing
L, Chiu CT, Liao HM, Chuang DM . Antidepressant mechanism of ketamine: perspective from preclinical studies. _Front Neurosci_ 2015; 9: 249. Article Google Scholar * Berk M, Malhi GS, Gray
LJ, Dean OM . The promise of N-acetylcysteine in neuropsychiatry. _Trends Pharmacol Sci_ 2013; 34: 167–177. Article CAS Google Scholar * Vandesompele J, De Preter K, Pattyn F, Poppe B,
Van Roy N, De Paepe A _et al_. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. _Genome Biol_ 2002; 3. Article Google
Scholar * Verhoeven AJ, Bolscher BG, Meerhof LJ, van Zwieten R, Keijer J, Weening RS _et al_. Characterization of two monoclonal antibodies against cytochrome b558 of human neutrophils.
_Blood_ 1989; 73: 1686–1694. CAS PubMed Google Scholar * Sorce S, Nuvolone M, Keller A, Falsig J, Varol A, Schwarz P _et al_. The role of the NADPH oxidase NOX2 in prion pathogenesis.
_PLoS Pathog_ 2014; 10: e1004531. Article Google Scholar * Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O'Keeffe S _et al_. An RNA-sequencing transcriptome and splicing database
of glia, neurons, and vascular cells of the cerebral cortex. _J Neurosci_ 2014; 34: 11929–11947. Article CAS Google Scholar * Seredenina T, Nayernia Z, Sorce S, Maghzal GJ, Filippova A,
Ling SC _et al_. Evaluation of NADPH oxidases as drug targets in a mouse model of familial amyotrophic lateral sclerosis. _Free Radical Biol Med_ 2016; 97: 95–108. Article CAS Google
Scholar * Behrens MM, Ali SS, Dao DN, Lucero J, Shekhtman G, Quick KL _et al_. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. _Science_ 2007;
318: 1645–1647. Article CAS Google Scholar * Behrens MM, Ali SS, Dugan LL . Interleukin-6 mediates the increase in NADPH-oxidase in the ketamine model of schizophrenia. _J Neurosci_ 2008;
28: 13957–13966. Article CAS Google Scholar * Webster MJ . Tissue preparation and banking. _Prog Brain Res_ 2006; 158: 3–14. Article CAS Google Scholar * Burritt JB, Fritel GN, Dahan
I, Pick E, Roos D, Jesaitis AJ . Epitope identification for human neutrophil flavocytochrome _b_ monoclonals 48 and 449. _Eur J Haematol_ 2000; 65: 407–413. Article CAS Google Scholar *
Roos D, de Boer M . Molecular diagnosis of chronic granulomatous disease. _Clin Exp Immunol_ 2014; 175: 139–149. Article CAS Google Scholar * Mittelbronn M, Dietz K, Schluesener HJ,
Meyermann R . Local distribution of microglia in the normal adult human central nervous system differs by up to one order of magnitude. _Acta Neuropathol_ 2001; 101: 249–255. CAS Google
Scholar * Kato TA, Yamauchi Y, Horikawa H, Monji A, Mizoguchi Y, Seki Y _et al_. Neurotransmitters, psychotropic drugs and microglia: clinical implications for psychiatry. _Curr Med Chem_
2013; 20: 331–344. CAS PubMed Google Scholar * Serrano F, Kolluri NS, Wientjes FB, Card JP, Klann E . NADPH oxidase immunoreactivity in the mouse brain. _Brain Res_ 2003; 988: 193–198.
Article CAS Google Scholar * Le Belle JE, Orozco NM, Paucar AA, Saxe JP, Mottahedeh J, Pyle AD _et al_. Proliferative neural stem cells have high endogenous ROS levels that regulate
self-renewal and neurogenesis in a PI3K/Akt-dependant manner. _Cell Stem Cell_ 2011; 8: 59–71. Article CAS Google Scholar * Bennett ML, Bennett FC, Liddelow SA, Ajami B, Zamanian JL,
Fernhoff NB _et al_. New tools for studying microglia in the mouse and human CNS. _Proc Natl Acad Sci USA_ 2016; 113: E1738–E1746. Article CAS Google Scholar * Dickinson BC, Peltier J,
Stone D, Schaffer DV, Chang CJ . Nox2 redox signaling maintains essential cell populations in the brain. _Nat Chem Biol_ 2011; 7: 106–112. Article CAS Google Scholar * Spaethling JM, Na
YJ, Lee J, Ulyanova AV, Baltuch GH, Bell TJ _et al_. Primary cell culture of live neurosurgically resected aged adult human brain cells and single cell transcriptomics. _Cell Rep_ 2017; 18:
791–803. Article CAS Google Scholar * Rustenhoven J, Aalderink M, Scotter EL, Oldfield RL, Bergin PS, Mee EW _et al_. TGF-beta1 regulates human brain pericyte inflammatory processes
involved in neurovasculature function. _J Neuroinflamm_ 2016; 13: 37. Article Google Scholar * Suvisaari J, Mantere O . Inflammation theories in psychotic disorders: a critical review.
_Infect Disord Drug Targets_ 2013; 13: 59–70. Article CAS Google Scholar * Steullet P, Cabungcal JH, Monin A, Dwir D, O'Donnell P, Cuenod M _et al_. Redox dysregulation,
neuroinflammation, and NMDA receptor hypofunction: a "central hub" in schizophrenia pathophysiology? _Schizophr Res_ 2016; 176: 41–51. Article CAS Google Scholar * Wang X,
Pinto-Duarte A, Sejnowski TJ, Behrens MM . How Nox2-containing NADPH oxidase affects cortical circuits in the NMDA receptor antagonist model of schizophrenia. _Antioxid Redox Signal_ 2013;
18: 1444–1462. Article CAS Google Scholar * Rosenberg G . The mechanisms of action of valproate in neuropsychiatric disorders: can we see the forest for the trees? _Cell Mol Life Sci_
2007; 64: 2090–2103. Article CAS Google Scholar * Gibbons HM, Smith AM, Teoh HH, Bergin PM, Mee EW, Faull RL _et al_. Valproic acid induces microglial dysfunction, not apoptosis, in human
glial cultures. _Neurobiol Dis_ 2011; 41: 96–103. Article CAS Google Scholar * Dragunow M, Greenwood JM, Cameron RE, Narayan PJ, O'Carroll SJ, Pearson AG _et al_. Valproic acid
induces caspase 3-mediated apoptosis in microglial cells. _Neuroscience_ 2006; 140: 1149–1156. Article CAS Google Scholar * Almodovar-Cuevas C, Navarro-Ruiz A, Bastidas-Ramirez BE,
Mora-Navarro MR, Garzon P . Valproic acid effects on leukocytes and platelets of Sprague–Dawley rats. _Gen Pharmacol_ 1985; 16: 423–426. Article CAS Google Scholar * Oedegaard KJ, Alda M,
Anand A, Andreassen OA, Balaraman Y, Berrettini WH _et al_. The pharmacogenomics of bipolar disorder study (PGBD): identification of genes for lithium response in a prospective sample. _BMC
Psychiatry_ 2016; 16: 129. Article Google Scholar * Nakagawa Y, Chiba K . Involvement of neuroinflammation during brain development in social cognitive deficits in autism spectrum
disorder and schizophrenia. _J Pharmacol Exp Ther_ 2016; 358: 504–515. Article CAS Google Scholar * Chen SK, Tvrdik P, Peden E, Cho S, Wu S, Spangrude G _et al_. Hematopoietic origin of
pathological grooming in Hoxb8 mutant mice. _Cell_ 2010; 141: 775–785. Article CAS Google Scholar * Prinz M, Priller J . Microglia and brain macrophages in the molecular age: from origin
to neuropsychiatric disease. _Nat Rev Neurosci_ 2014; 15: 300–312. Article CAS Google Scholar * Bilimoria PM, Stevens B . Microglia function during brain development: new insights from
animal models. _Brain Res_ 2015; 1617: 7–17. Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We are grateful to Prof Maree Webster, Prof Alexandre Dayer and Dr Michel
Dubois-Dauphin for helpful discussions; to Christelle Barraclough and Didier Chollet for the assistance with qPCR and to Monika Bieri, Francois Prodon and Olivier Brun for the assistance
with the image acquisition and analysis. This study was funded by by the European Community's Framework Programme (FP7/2007–2013) under Grant 278611 (Neurinox) and the The State
Secretariat for Education, Research and Innovation under Grant C13.0142. AUTHOR INFORMATION Author notes * T Seredenina, S Sorce, V Jaquet and K-H Krause: These authors contributed equally
to this work. AUTHORS AND AFFILIATIONS * Department of Pathology and Immunology, Centre Médical Universitaire, University of Geneva, Geneva, Switzerland T Seredenina, X-J Ma Mulone, O
Plastre, V Jaquet & K-H Krause * Institute of Neuropathology, University Hospital of Zurich, Zurich, Switzerland S Sorce & A Aguzzi * Department of Internal Medicine, Rehabilitation
and Geriatrics, Geneva University Hospitals, Geneva, Switzerland F R Herrmann * Department of Genetic and Laboratory Medicine, Geneva University Hospitals, Geneva, Switzerland K-H Krause
Authors * T Seredenina View author publications You can also search for this author inPubMed Google Scholar * S Sorce View author publications You can also search for this author inPubMed
Google Scholar * F R Herrmann View author publications You can also search for this author inPubMed Google Scholar * X-J Ma Mulone View author publications You can also search for this
author inPubMed Google Scholar * O Plastre View author publications You can also search for this author inPubMed Google Scholar * A Aguzzi View author publications You can also search for
this author inPubMed Google Scholar * V Jaquet View author publications You can also search for this author inPubMed Google Scholar * K-H Krause View author publications You can also search
for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to V Jaquet. ETHICS DECLARATIONS COMPETING INTERESTS VJ and KHK hold shares in Genkyotex SA, a company developing
NOX inhibitors. The remaining authors declare no conflict of interests. ADDITIONAL INFORMATION Supplementary Information accompanies the paper on the _Translational Psychiatry_ website
SUPPLEMENTARY INFORMATION SUPPLEMENTARY TABLES (DOCX 19 KB) SUPPLEMENTARY FIGURE 1 (JPG 5808 KB) SUPPLEMENTARY FIGURE 2 (JPG 4794 KB) SUPPLEMENTARY FIGURE 3 (JPG 1559 KB) RIGHTS AND
PERMISSIONS This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included
in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain
permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/ Reprints and permissions ABOUT THIS
ARTICLE CITE THIS ARTICLE Seredenina, T., Sorce, S., Herrmann, F. _et al._ Decreased NOX2 expression in the brain of patients with bipolar disorder: association with valproic acid
prescription and substance abuse. _Transl Psychiatry_ 7, e1206 (2017). https://doi.org/10.1038/tp.2017.175 Download citation * Received: 20 March 2017 * Revised: 05 May 2017 * Accepted: 13
June 2017 * Published: 15 August 2017 * Issue Date: August 2017 * DOI: https://doi.org/10.1038/tp.2017.175 SHARE THIS ARTICLE Anyone you share the following link with will be able to read
this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative