Isatuximab, carfilzomib, lenalidomide, and dexamethasone (isa-krd) in front-line treatment of high-risk multiple myeloma: interim analysis of the gmmg-concept trial
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

The continuous implementation of novel agents in the treatment of multiple myeloma (MM) has led to significant improvement in survival. Especially the addition of monoclonal antibodies
directed against CD38 to standard of care regimens led to significantly deepening responses and improved survival outcomes [1]. However, treatment of high-risk (HR) MM remains challenging
with still markedly impaired survival, and risk-adapted treatment concepts are rare [2, 3]. Even aggressive approaches resulted in two-year median progression-free survival (PFS) rates of
approximately 50% [4]. The GMMG-CONCEPT trial (NCT03104842) investigates the quadruplet regimen isatuximab, carfilzomib, lenalidomide, and dexamethasone (Isa-KRd) in front-line treatment of
solely HRMM. Here, we report the interim analysis (IA) focusing on best response during induction and presenting first data on PFS of the first 50 patients. The IA reports on the first 50
patients included in this phase II, open-label, two-arm, multi-center clinical trial with planned recruitment of 246 patients. Patients were eligible if they had ND symptomatic MM according
to international consensus criteria with HR features, defined by the presence of del17p (≥10% of purified cells) or t(4;14) or t(14;16) or > 3 copies of 1q21. Furthermore, all patients
had to have ISS II or III stage disease [5]. Prior MM-specific treatment was allowed as emergency treatment with a maximum of one cycle of any anti-MM first-line treatment. All patients
received ECG and ECHO at screening. Patients were openly assigned to study arms according to age and transplant eligibility (arm A: patients ≤ 70 years and eligible for HDT; arm B: patients
> 70 years). Study treatment consisted of six cycles Isa-KRd induction, four cycles Isa-KRd consolidation, and Isa-KR maintenance. Transplant-eligible patients underwent HDT with
autologous stem cell transplantation (ASCT) after stem cell collection, transplant-ineligible patients received two additional Isa-KRd induction cycles. Primary endpoint of this trial is
achievement of minimal residual disease (MRD) negativity measured by next-generation flow after consolidation. Induction treatment with Isa-KRd consisted of six 28-day-cycles with isatuximab
10 mg/kg of body weight intravenously (i.v.) weekly during the first and on day 1 and 15 of any subsequent cycle, carfilzomib 20 mg/m2 of body surface area i.v. on day 1 and 2 of the first
and 36 mg/m2 i.v. on day 8, 9, 15, 16 of the first and day 1, 2, 8, 9, 15, 16 of any subsequent cycle, lenalidomide 25 mg orally (p.o.) on day 1–21 of all cycles, and dexamethasone 40 mg (20
mg for subjects >75 years of age) p.o./i.v. on day 1,8,15,22 of all cycles. Prophylactic anticoagulation was obligate and chosen upon the investigator’s decision. The population for this
IA on overall response rate (ORR) at the end of induction includes the first 50 enrolled patients who received at least one cycle of induction treatment and were eligible for at least one
response assessment (46 patients in arm A and 4 patients in arm B). Overall response was determined as the best response until the end of induction including mobilization. Median age was 58
(range: 42–82) years. 56% of patients showed ISS stage II, 44% ISS stage III disease. The most common cytogenetic aberration defining HR disease was del17p in 52% of patients followed by
>3 copies of 1q21 in 42%, t(4;14) in 38% and t(14;16) in 12%, respectively. 15 patients (30%) showed ≥2 HR aberrations and 20% of patients had an elevated LDH. Forty-four of 50 patients
completed induction, seven patients discontinued treatment due to progressive disease (_n_ = 3), death (_n_ = 3) or patient’s request (_n_ = 1). Average dose intensities were 95.7% for
isatuximab, 95.2% for dexamethasone, 91.6% for carfilzomib, and 87.9% for lenalidomide. With regards to the goal of this IA reporting on best response during induction, all patients (50/50;
ORR = 100%) responded to the induction treatment showing at least a partial response (PR) as best response. 45/50 patients (90%) showed a VGPR or better, 20/50 patients (40%) a complete
response (CR) and three patients (6%) a stringent complete response (sCR) (Table 1). Of the four patients in treatment arm B, all patients completed induction and achieved VGPR (Table 1).
Median time to first response was 34 days with 95.8% achieving ≥PR after the first induction cycle. Assessment of MRD during induction was recommended in all patients achieving ≥VGPR. In
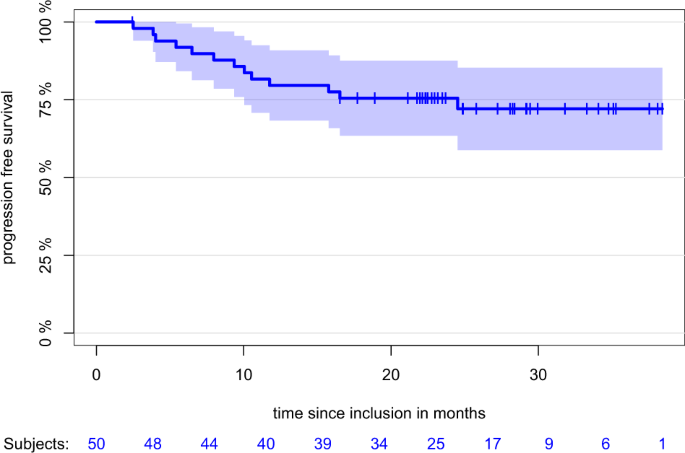
total, 33 patients underwent MRD assessment. Of those, 20 patients were negative, 11 patients positive, two patients were non-assessable. After a median follow-up of 24.9 months, median PFS
was not reached with a median 12-month PFS of 79.6% (CI: 68.3%; 90.9%) and a median 24-month PFS of 75.5% (CI: 63.5%; 87.6%) (Fig. 1). Most common adverse events (AE) of any grade occurring
in ≥ 10% of patients were neutropenia, lymphopenia, leukopenia, anemia, thrombocytopenia, upper respiratory tract infections, pyrexia, rash, peripheral sensory neuropathy, arterial
hypertension, and nasopharyngitis. Most common AEs grade 3/4 occurring in ≥10% of patients were neutropenia, lymphopenia, leukopenia, thrombocytopenia, anemia, infections, and arterial
hypertension. Serious adverse events (SAE) of ≥grade 3 occurred in 18 patients, most common SAEs being infectious (_n_ = 5) and cardiovascular disorders (_n_ = 5). Grade 3/4 cardiac failure
was documented in 4 patients, isatuximab-related infusion reactions occured in 32%, all grade 1 or 2. Death on study during induction phase including mobilization occurred in three patients
with two fatal pneumonias and one fatal neutropenic sepsis after stem cell mobilization. Median number of collected CD34 + cells was 6.0 × 106 per kg body weight. Trials for solely HRMM are
rare and the proportion of HR patients in first-line phase III trials is generally limited representing around 15–25% of the total patient population [6, 7]. Even more, a substantial
proportion of ultra HR patients does not enter clinical trials due to aggressiveness of the disease leading to emergency treatment before potential trial inclusion. With one cycle of any
myeloma-directed therapy being allowed before enrollment, the GMMG-CONCEPT trial enabled even ultra HR patients including plasma cell leukemia and patients primary non-responding to a first
treatment cycle to be included. GMMG-CONCEPT is the first trial investigating the Isa-KRd quadruplet regimen in the treatment of MM. This IA focusing on best overall response during
induction showed an ORR of 100% with 90% of patients achieving ≥ VGPR and 46% showing a CR or sCR and so revealed promising results with no patients primary refractory to the chosen
quadruplet combination. MRD analysis during induction was not obligate, however, recommended for all patients achieving at least a VGPR. Of 33 patients tested for MRD at this early time
point, 31 were evaluable and of those, 20 were negative for MRD. To address the question whether the achieved early high response rates translate into survival outcome, we conducted a PFS
analysis after a median follow-up of 24.9 months demonstrating a two-year PFS rate of 75.5% with a median PFS not reached. To the best of our knowledge, this is one of the highest described
in this unfavorable patient group. Isa-KRd as a quadruplet regimen was tolerable, AEs were clinically manageable and consistent with known toxicities of each individual substance. Reported
AEs of interest, especially cardiac toxicities, were within the expected range, rates of peripheral neuropathy were low. Regarding the 3 reported fatal infectious events, with one where a
relation to study medication could not be excluded, a careful look on the larger patient population is needed. However, in this difficult-to-treat population, we see a positive risk-benefit
analysis outweighing efficacy above toxicity. Currently, there are several trials underway investigating quadruplet regimens in NDMM and even HRMM. In the SWOG 1211 trial for HR patients,
addition of the monoclonal anti-SLAMF7 antibody elotuzumab to bortezomib, lenalidomide and dexamethasone did not lead to improved outcome [8]. ORR was 83% with a 2.1% CR and a 21.3% VGPR
rate in the quadruplet treatment arm, median PFS was 31.47 months [8]. Out of the FORTE trial, Gay and colleagues reported a PFS rate of 62% after four years in HR patients treated with
upfront KRd, ASCT, and KR or R maintenance, however using a broad definition of HR accounting for more than 50% of the trial population [9]. The UK OPTIMUM HR study investigating quintruplet
induction of Dara-CVRD followed by HDT and ASCT in HR patients most recently reported an ORR of 94% with a ≥ VGPR rate of 80% as the best response during induction [10]. Two recent trials
are investigating the anti-CD38-KRd (Dara-KRd) combination not restricted to HR patients: The single-center MANHATTAN trial reported an ORR of 100% with a 1-year-PFS of 98% in 41 patients
[11]. The MASTER trial showed a rate of 90% ≥VGPR after induction in 70 patients [12]. Taken together, anti-CD38-KRd trials particularly underline the high potential in achieving deep
responses including MRD-negativity. This might open again the discussion about the future relevance of primary HDT and ASCT. In summary, our data demonstrate encouraging rates of rapid and
deep remissions in HR MM patients with Isa-KRd induction, which may translate into durable responses in this difficult-to-treat patient group and is supported by the first survival data on
PFS with a two-year PFS rate of 75.5%. The trial completed recruitment of the first population of 153 patients early in 2020 and is ongoing with an expansion cohort for a total of 246
patients. Further results will be reported. REFERENCES * Kumar SK, Rajkumar V, Kyle RA, van Duin M, Sonneveld P, Mateos MV, et al. Multiple myeloma. Nat Rev Dis Prim. 2017;3:17046
https://doi.org/10.1038/nrdp.2017.46. PMID: 28726797 Article PubMed Google Scholar * Nandakumar B, Binder M, Dispenzieri A, Kapoor P, Buadi F, Gertz MA, et al. Continued improvement in
survival in multiple myeloma (MM) including high-risk patients. JCO. Am Society of Clin Oncol. 2019;37:8039–9. * Kazmi SM, Nusrat M, Gunaydin H, Cornelison AM, Shah N, Kebriaei P, et al.
Outcomes among high-risk and standard-risk multiple myeloma patients treated with high-dose chemotherapy and autologous hematopoietic stem-cell transplantation. Clin Lymphoma Myeloma Leuk.
2015;15:687–93. https://doi.org/10.1016/j.clml.2015.07.641. Epub 2015 Aug 5. PMID: 26361647; PMCID: PMC4644689 Article PubMed PubMed Central Google Scholar * Nair B, van Rhee F,
Shaughnessy JD Jr, Anaissie E, Szymonifka J, Hoering A, et al. Superior results of Total Therapy 3 (2003-33) in gene expression profiling-defined low-risk multiple myeloma confirmed in
subsequent trial 2006-66 with VRD maintenance. Blood 2010;115:4168–73. https://doi.org/10.1182/blood-2009-11-255620. Epub 2010 Feb 2. PMID: 20124509; PMCID: PMC2879104 Article CAS PubMed
PubMed Central Google Scholar * Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos M-V, et al. International Myeloma Working Group updated criteria for the diagnosis of
multiple myeloma. Lancet Oncol. 2014;15:e538–48. Article Google Scholar * Mateos MV, Dimopoulos MA, Cavo M, Suzuki K, Jakubowiak A, Knop S, et al. Daratumumab plus bortezomib, melphalan,
and prednisone for untreated myeloma. N Engl J Med. 2018;378:518–28. * Moreau P, Attal M, Hulin C, Arnulf B, Belhadj K, Benboubker L, et al. Bortezomib, thalidomide, and dexamethasone with
or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): a randomised, open-label, phase 3 study. Lancet
2019;394(Jul):29–38. Article CAS Google Scholar * Usmani SZ, Hoering A, Ailawadhi S, Sexton R, Lipe B, Hita SF, et al. SWOG1211 Trial Investigators. Bortezomib, lenalidomide, and
dexamethasone with or without elotuzumab in patients with untreated, high-risk multiple myeloma (SWOG-1211): primary analysis of a randomised, phase 2 trial. Lancet Haematol.
2021;8(Jan):e45–e54. https://doi.org/10.1016/S2352-3026(20)30354-9. Epub 2020 Dec 22. PMID: 33357482 Article PubMed Google Scholar * Mina R, Zamagni E, Fazio F, Ledda A, Palmas A, Aquino
S, et al. Efficacy of carfilzomib-based induction/consolidation with or without autologous transplant and lenalidomide or carfilzomib-lenalidomide maintenance in high-risk patients in the
forte trial, HemaSphere, 2021;5:(S2). EHA 2021 Abstract S182. * Kaiser M, Hall A, Walker K, de Tute R, Sadie R, Ingleson E, et al. Depth of response and mrd status in ultra high-risk myeloma
and plasma cell leukemia treated with dara-cvrd and augmented autologous transplant: results of the risk-stratified uk optimum/muknine trial, HemaSphere, 2021;5:(S2). EHA 2021 Abstract
S181. * Landgren O, Hultcrantz M, Diamond B, Lesokhin AM, Mailankody S, Hassoun H, et al. Safety and effectiveness of weekly carfilzomib, lenalidomide, dexamethasone, and daratumumab
combination therapy for patients with newly diagnosed multiple myeloma: the MANHATTAN nonrandomized clinical trial. JAMA Oncol. 2021;7(Jun):862–8.
https://doi.org/10.1001/jamaoncol.2021.0611. PMID: 33856405; PMCID: PMC8050789. Article PubMed Google Scholar * Costa L, Chhabra S, Godby K, et al. Daratumumab, carfilzomib, lenalidomide
and dexamethasone (Dara-KRd) induction, autologous transplantation and post-transplant, response-adapted, measurable residual disease (MRD)-based Dara-Krd consolidation in patients with
newly diagnosed multiple myeloma (NDMM). Blood 2019;134(Supplement_1):860. https://doi.org/10.1182/blood-2019-123170. Download references ACKNOWLEDGEMENTS We thank the patients who consented
to participate in this clinical trial and the clinical research teams at the participating centers. We are grateful to Philippe Moreau, Nikhil Munshi and Lutz Eder of the Data Monitoring
Committee for their work in supporting this study. The trial was sponsored by the University Medical Center Hamburg-Eppendorf. Study drug and financial support by Amgen, Celgene | A Bristol
Myers Squibb Company and Sanofi. FUNDING Open Access funding enabled and organized by Projekt DEAL. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Hematology, Oncology and Bone
Marrow Transplantation with Section of Pneumology, University Medical Center Hamburg-Eppendorf, Hamburg, Germany Lisa B. Leypoldt, Anne Marie Asemissen, Carsten Bokemeyer & Katja C.
Weisel * Department of Hematology, Oncology, Immunology, Rheumatology and Pulmonology, University Hospital of Tuebingen, Tuebingen, Germany Britta Besemer * Department of Hematology,
Oncology and Bone Marrow Transplantation, Klinikum Chemnitz, Chemnitz, Germany Mathias Hänel * Department of Internal Medicine, Charité University Medicine Berlin, Berlin, Germany Igor
Wolfgang Blau * Department of Hematology, Oncology and Palliative Care, Klinikum Bielefeld Mitte, Bielefeld, Germany Martin Görner * Department of Internal Medicine, Hematology and Oncology,
Johanniter Krankenhaus Bonn, Bonn, Germany Yon-Dschun Ko * Department of Hematology and Stem Cell Transplantation, University Hospital Essen, University Duisburg-Essen, German Cancer
Consortium (DKTK partner site Essen), Essen, Germany Hans Christian Reinhardt * Department of Hematology and Oncology, St. Antonius Hospital Eschweiler, Eschweiler, Germany Peter Staib *
Department of Hematology, Oncology and Immunology, University Hospital of Gießen and Marburg, Marburg, Germany Christoph Mann * University Hospital Heidelberg, Internal Medicine V and
National Center for Tumor Diseases (NCT), Heidelberg, Germany Raphael Lutz & Hartmut Goldschmidt * Department of Internal Medicine III, University Medical Center Mainz, Mainz, Germany
Markus Munder * Department of Hematology, Oncology and Gastroenterology, Maria Hilf Kliniken, Mönchengladbach, Germany Ullrich Graeven * Department of Oncology, Hematology and Stem Cell
Transplantation, Klinikum Osnabrück, Osnabrück, Germany Rudolf Peceny * Asklepios Tumorzentrum Hamburg, AK Altona and AK St. Georg, Hamburg, Germany Hans Salwender * Institute of Human
Genetics, University of Heidelberg, Heidelberg, Germany Anna Jauch * Center for Clinical Trials, University Hospital of Tuebingen, Tuebingen, Germany Manola Zago * Division of Biostatistics,
German Cancer Research Center (DKFZ) Heidelberg, Heidelberg, Germany Axel Benner & Diana Tichy Authors * Lisa B. Leypoldt View author publications You can also search for this author
inPubMed Google Scholar * Britta Besemer View author publications You can also search for this author inPubMed Google Scholar * Anne Marie Asemissen View author publications You can also
search for this author inPubMed Google Scholar * Mathias Hänel View author publications You can also search for this author inPubMed Google Scholar * Igor Wolfgang Blau View author
publications You can also search for this author inPubMed Google Scholar * Martin Görner View author publications You can also search for this author inPubMed Google Scholar * Yon-Dschun Ko
View author publications You can also search for this author inPubMed Google Scholar * Hans Christian Reinhardt View author publications You can also search for this author inPubMed Google
Scholar * Peter Staib View author publications You can also search for this author inPubMed Google Scholar * Christoph Mann View author publications You can also search for this author
inPubMed Google Scholar * Raphael Lutz View author publications You can also search for this author inPubMed Google Scholar * Markus Munder View author publications You can also search for
this author inPubMed Google Scholar * Ullrich Graeven View author publications You can also search for this author inPubMed Google Scholar * Rudolf Peceny View author publications You can
also search for this author inPubMed Google Scholar * Hans Salwender View author publications You can also search for this author inPubMed Google Scholar * Anna Jauch View author
publications You can also search for this author inPubMed Google Scholar * Manola Zago View author publications You can also search for this author inPubMed Google Scholar * Axel Benner View
author publications You can also search for this author inPubMed Google Scholar * Diana Tichy View author publications You can also search for this author inPubMed Google Scholar * Carsten
Bokemeyer View author publications You can also search for this author inPubMed Google Scholar * Hartmut Goldschmidt View author publications You can also search for this author inPubMed
Google Scholar * Katja C. Weisel View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS LL, KW, AB, DT, HG, AJ, MZ, and CB participated in the
conception and design of the study. All authors participated in the analysis and interpretation of data, the writing of the manuscript and the decision to submit for publication. Patient
data were collected by LL, AMA, BB, MH, IWB, MG, Y-DK, HCR, PS, CM, RL, MM, UG, RP, HS, CB, HG, and KW. CORRESPONDING AUTHOR Correspondence to Katja C. Weisel. ETHICS DECLARATIONS COMPETING
INTERESTS Dr. Leypoldt reports grants and non-financial support from Celgene | A Bristol Myers Squibb Company, grants and non-financial support from Sanofi, grants and non-financial support
from Amgen, during the conduct of the study; non-financial support from GSK, non-financial support from Abbvie, outside the submitted work; Dr. Asemissen has nothing to disclose. Dr. Besemer
has nothing to disclose. Dr. Hänel reports personal fees from Celgene | A Bristol Myers Squibb Company, personal fees from Novartis, personal fees from Takeda, personal fees from Amgen,
during the conduct of the study; Dr. Blau has nothing to disclose. Dr. Görner has nothing to disclose. Dr. Ko has nothing to disclose. Dr. Reinhardt reports personal fees from Abbvie, grants
from Gilead, personal fees from Merck, other from CDL Therapeutics GmbH, outside the submitted work; Dr. Staib reports grants, personal fees, non-financial support and other from Abbvie,
grants, personal fees, non-financial support and other from Amgen, grants, personal fees, non-financial support and other from Celgene | A Bristol Myers Squibb Company, grants, personal
fees, non-financial support and other from Janssen-Cilag, grants, personal fees, non-financial support and other from Novartis, grants, personal fees, non-financial support and other from
Gilead, grants, personal fees, non-financial support and other from Pfizer, grants, personal fees, non-financial support and other from Roche, outside the submitted work; Dr. Mann has
nothing to disclose. Dr. Lutz has nothing to disclose. Dr. Munder reports personal fees and non-financial support from Janssen, personal fees and non-financial support from Amgen, grants
from Incyte, personal fees and non-financial support from BMS, personal fees from Abbvie, personal fees from Sanofi, personal fees from GSK, personal fees from Takeda, outside the submitted
work; Dr. Graeven reports personal fees from Amgen, personal fees and non-financial support from Boehringer Ingelheim, personal fees from Daichi Sankyo, personal fees from Servier, personal
fees from Celgene | A Bristol Myers Squibb Company, personal fees from Astra Zeneca, personal fees from Johnson Johnson, non-financial support from Merck, personal fees from MSD, personal
fees from BMS, during the conduct of the study; Dr. Peceny reports grants and personal fees from Sanofi Genzyme, grants from Novartis, grants from DRK Blutspendedienst NSTOB, grants from
Boehringer Ingelheim Pharma GmbH & Co KG, grants from Celgene | A Bristol Myers Squibb Company, outside the submitted work; Dr. Salwender reports personal fees from Bristol-Myers
Squibb/Celgene, personal fees from Janssen Cilag, personal fees from Glaxo Smith Kline, personal fees from Oncopeptides, personal fees from Takeda, personal fees from Sanofi, personal fees
from AbbVie, personal fees from Amgen, outside the submitted work; Dr. Jauch has nothing to disclose. Dr. Zago has nothing to disclose. Axel Benner has nothing to disclose. Dr. Tichy has
nothing to disclose. Dr. Bokemeyer reports personal fees from Sanofi Aventis, personal fees from Merck KgA, personal fees from Bristol-Myers Squibb, personal fees from Merck Sharp &
Dohme, personal fees from Lilly Imclone, personal fees from Bayer Healthcare, personal fees from GSO Contract research, personal fees from AOK-Rheinland-Hamburg, personal fees from Novartis,
outside the submitted work; Dr. Goldschmidt reports grants, personal fees, non-financial support and other from Amgen, grants, personal fees, non-financial support and other from BMS,
grants, personal fees, non-financial support and other from Celgene, grants, personal fees, and other from Chugai, grants, personal fees, non-financial support and other from Janssen,
grants, personal fees, non-financial support and other from Sanofi, other from Incyte, other from Molecular Partners, other from Merck Sharp and Dohme (MSD), other from Mundipharma, grants,
personal fees, non-financial support and other from Takeda, personal fees and other from Novartis, personal fees from Adaptive Biotechnology, personal fees from GlaxoSmithKline (GSK),
outside the submitted work. Dr. Weisel reports grants from AMGEN, grants from Celgene | A Bristol Myers Squibb Company, grants from Sanofi, during the conduct of the study; grants, personal
fees and non-financial support from Amgen, personal fees and non-financial support from BMS, grants, personal fees and non-financial support from Celgene | A Bristol Myers Squibb Company,
personal fees from Adaptive Biotech, grants, personal fees and non-financial support from Janssen, personal fees and non-financial support from GSK, personal fees from Karyopharm, grants,
personal fees and non-financial support from Sanofi, personal fees and non-financial support from Takeda, personal fees from Oncopeptides, personal fees from Roche, outside the submitted
work. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. RIGHTS AND PERMISSIONS
OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or
format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or
other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in
the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the
copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Leypoldt, L.B., Besemer,
B., Asemissen, A.M. _et al._ Isatuximab, carfilzomib, lenalidomide, and dexamethasone (Isa-KRd) in front-line treatment of high-risk multiple myeloma: interim analysis of the GMMG-CONCEPT
trial. _Leukemia_ 36, 885–888 (2022). https://doi.org/10.1038/s41375-021-01431-x Download citation * Received: 28 June 2021 * Revised: 14 September 2021 * Accepted: 16 September 2021 *
Published: 03 November 2021 * Issue Date: March 2022 * DOI: https://doi.org/10.1038/s41375-021-01431-x SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative